We report our experience with the surgical treatment of anomalous origin of the left pulmonary artery in eight children between 2004 and 2009. The congenital heart disease most frequently associated with this condition was patent ductus arteriosus. Surgery was carried out with extracorporeal circulation in five children, and without, in three. The anomalous pulmonary artery was divided and translocated to the main pulmonary artery. One patient died soon after surgery because of hemodynamic instability and another died later because of respiratory complications. The other patients progressed satisfactorily during follow-up: the reimplanted artery remained patent in all cases and respiratory symptoms improved. However, one patient required endoscopic treatment.
Keywords
Anomalous origin of left pulmonary artery (LPA), or pulmonary artery (PA) sling, is a rare malformation in which the LPA stems from the posterior portion of right pulmonary artery, continues toward the left side and positions itself between the trachea and the esophagus. The anomalous artery, frequently hypoplastic, compresses the esophagus anteriorly and the trachea posteriorly.1 The prevalence could be 1 of every 17,000 school-aged children,2 although there are no data concerning infants, the largest and most severely affected group of patients. It produces respiratory symptoms due to airway obstruction, often acutely and with fatal consequences.
The treatment requires surgery, with disinsertion of the anomalous LPA and its reimplantation into the main PA. In cases of significant tracheal stenoses, different surgical procedures can be performed.3,4,5
MethodsBetween January 2004 and November 2009, eight children were treated surgically. One of the patients had been referred to us prior to this period.6
Table 1 shows the characteristics of the patients. Most of them were under one year of age at the time of surgery. All of the patients presented clinically with respiratory distress and one had associated heart failure due to the accompanying heart defects. The associated anomalies are listed in Table 1.
Table 1. Patient Characteristics
| Age | Sex | Clinical signs | Associated anomalies | Age and weight at time of surgery | Surgical procedure | Duration surgery/ECC | Postoperative outcome | |
| 1 | 5 months | M | Respiratory distress. Stridor | 7 months, 10 kg | Median sternotomy. Disinsertion of LPA and anastomosis to main PA. Reconstruction at origin of right main bronchus | 3 h and 40’/95’ | Patency of LPA. Mechanical ventilation, 5 days | |
| 2 | 3.5 months | M | Respiratory distress. I/VI systolic murmur. | Patent ductus arteriosus | 4 months, 6 kg | Median sternotomy. Section of the ductus, disinsertion of LPA and anastomosis to main PA | 2 h and 30’/65’ | Patency of LPA. Mechanical ventilation, 9 days |
| 3 | 11 months | M | Asymptomatic | Persistent LSVC | 21 months, 12.9 kg | Median sternotomy. Disinsertion of LPA and anastomosis to main PA | 2 h and 25’/73’ | Patency of LPA. Immediate postoperative extubation |
| 4 | 11 months | F | Respiratory distress. Stridor. | Patent ductus arteriosus | 11 months, 11 kg | Median sternotomy. Section of the ductus, disinsertion of LPA and anastomosis to main PA | 2 h and 45’/50’ | Patency of LPA. Mechanical ventilation, 19 days |
| 5 | 1 month | M | Respiratory distress. III/VI systolic murmur. | ASD. VSD. Patent ductus arteriosus. Multiple malformation syndrome | 1.5 months, 4 kg | Left thoracotomy. Section of the ductus, disinsertion of LPA and anastomosis to main PA | 1 h and 25’/without ECC | Patency of LPA. Intermittent mechanical ventilation and noninvasive ventilation for first 6 postoperative weeks. Tracheal stenosis with two dilatations |
| 6 | 2 months | M | Respiratory distress. Heart failure. | ASD. Patent ductus arteriosus. Right upper lobe tracheal bronchus | 2 months, 3.5 kg | Left thoracotomy. Section of the ductus, disinsertion of LPA and anastomosis to main PA | 1 h and 55’/without ECC | Patency of LPA. Mechanical ventilation, 1 day |
| 7 | 3 months | M | Respiratory stress. Stridor. | Patent ductus arteriosus | 3 months 1 week, 5 kg | Median sternotomy. Section of the ductus, disinsertion of LPA and anastomosis to main PA | 2 h and 35’/44’ | Patency of LPA. Mechanical ventilation, 87 days. Tracheal stenosis with 3 dilatations, tracheal stent. Death |
| 8 | 3 days | F | Asymptomatic | Dextrocardia. Right upper lobe tracheal bronchus. Patent ductus arteriosus | 1 month and 11 days, 2 kg | Left thoracotomy. Section of the ductus, disinsertion of LPA and anastomosis to main PA | 1 h and 05’/without ECC | Patency of LPA. Mechanical ventilation, 1 day. Death |
ASD, atrial septal defect; ECC, extracorporeal circulation; F, female; LPA, left pulmonary artery; LSVC, left superior vena cava connected to coronary sinus; M, male; PA, main pulmonary artery; VSD, ventricular septal defect.
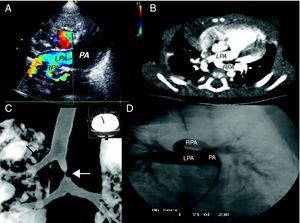
In all of the patients, the suspicion was based on an echocardiogram and was confirmed by computed tomography angiography (CTA) and/or magnetic resonance (MR) (Figure 1). One patient underwent diagnostic catheterization. CTA enabled us to define the concomitant tracheobronchial anomalies. All of the patients had tracheal stenosis: there were five cases of segmental stenosis with accompanying laryngotracheomalacia and three cases of long stenosis with complete tracheal rings; the autopsy of one of the latter children revealed left bronchial atresia. Two of the patients had right upper lobe tracheal bronchus (bronchus suis).
Figure 1. A: Echocardiogram showing the aberrant origin of left pulmonary artery, which stems from right pulmonary artery. B: computed tomography; the black arrow indicates the trachea. C: computed tomography showing right tracheal bronchus (black arrow) and tracheal stenosis (white arrow). D: pulmonary angiography; LPA, left pulmonary artery; PA, main pulmonary artery; RPA, right pulmonary artery.
The mean duration of follow-up was 30.5 months (range 12 to 67 months).
ResultsSurgical repair was carried out in all the patients, with two deaths.
The mean age at diagnosis was 4.6 months (range, 3 days to 11 months). The mean age of the first four patients was 7.6 months and that of the last four was 1.5 months, considerably younger, perhaps due to a greater sensitivity for detection. In the last patient, the diagnosis was reached three days after birth and based on a prenatal study that revealed the presence of dextrocardia.
At the time of surgery, the mean age of the patients was 5.5 months (range, one month 11 days to 21 months). The mean age of the last four patients was two months, versus 10.7 months for the first four.
In five patients, the procedure involved median sternotomy and extracorporeal circulation (ECC); disinsertion of the anomalous LPA was carried out, the retrotracheal space was freed and direct anastomosis to the left side of the main PA was performed. In three, in addition, the ductus was sectioned. One of the patients in this group died 87 days after the operation due to respiratory problems secondary to severe tracheal stenosis that did not respond to dilatations or the implantation of a tracheal stent.
In three patients, the smallest infants who were also in the poorest clinical condition, access was achieved via left thoracotomy; the surgical technique was that described above. One patient had a perimembranous ventricular septal defect measuring 3mm and an atrial septal defect (ASD) measuring 8mm, which were managed with medical treatment with a good response. Another patient had a small ASD that was not considered significant. The third patient died. He had developed marked hemodynamic and respiratory instability prior to the operation, with several cardiorespiratory arrests. Following the intervention, the patency of the reimplanted artery was verified, but the infant died 12hours later.
The mean operating time was 137.5min (range, 65 to 220min). The duration of ECC, when employed, was 64min (range: 44 to 95min).
The follow-up echocardiograms showed patency of the reimplanted artery in every case, making therapeutic procedures unnecessary.
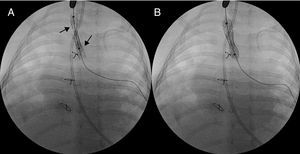
All the patients had tracheal stenosis, which improved after surgery except in two patients, who were very young infants with very severe stenoses, in whom the course was indolent and prolonged mechanical ventilation and endoscopic techniques with tracheal dilatation were necessary. In one patient (case no. 5), two dilatations were performed that resulted in an improvement in the respiratory function that permitted extubation. Another patient (case no. 7) required three dilatations and the placement of two tracheal stents (6×22mm and 7×22mm, Numed®) (Figure 2); he died three months after the operation due to respiratory complications. In the remaining patients, there are no records of hospital admissions due to tracheobronchial complaints. Residual stridor has resolved in all cases and one patient has swallowing problems due to paralysis of the left vocal chord secondary to paralysis of left recurrent laryngeal nerve.
Figure 2. A: double stent in the trachea (black arrows). B: final position with expanded stents.
DiscussionAnomalous LPA is produced by an abnormal involution of the proximal portion of the left sixth aortic arch. The heart defects most frequently associated with it are patent ductus arteriosus, septal defects and persistent left superior vena cava connected to the coronary sinus. The associated noncardiac abnormalities include tracheal stenosis, right tracheal bronchus and underdeveloped right lung.7
The diagnosis is suspected on the basis of the echocardiographic images, whereas spiral computed tomography with three-dimensional reconstruction and MR angiography can help to verify the tracheobronchial anomalies and the presence of complete tracheal rings.8
Surgical treatment is required and is carried out by means of one of two techniques. One consists of the division of the LPA, with anterior translocation to the trachea and reimplantation. The technique involves thoracotomy without ECC or median sternotomy with cardiopulmonary bypass. The latter facilitates the dissection of the LPA and its reimplantation, reduces tension in the anastomoses3 and enables the repair of intracardiac defects and tracheal rings, if necessary.3,9 This was the technique used in our patients.
The second option consists of the resection and anastomosis of the tracheal stenosis with reinsertion of anterior LPA into the trachea, its passage between the two ends of the divided trachea, with median sternotomy and ECC.1 It produces tracheal compression and kinking of the reimplanted artery1,5 and cannot be employed in the presence of significant tracheal stenosis.3
The treatment of tracheal stenosis will depend on the severity, and is a subject of debate, especially considering that there are questions concerning the growth of circumferential tracheal anastomoses in newborn infants and children. In short stenoses, the reimplantation of LPA suffices and it is not necessary to intervene in the tracheal stenosis. In long-segment stenoses, the techniques include tracheoplasty using autologous material and slide tracheoplasty.3,10,11 The former involves tracheal reconstruction with a pericardial patch or a costal cartilage graft, and it offers the advantage of potential growth, but the disadvantages are higher rates of perioperative mortality and respiratory complications. Slide tracheoplasty reconstructs the trachea with the patient's own tracheal tissue and allows the growth of the repaired segments, with a lesser tendency toward the development of granulation tissue, rapid reepithelialization and shorter mechanical ventilation times.
Some groups have employed endoscopic techniques with dilatation or a stent, with good results in congenital tracheal stenosis.12
The mortality reported varies according to the series. In two of the most representative ones, it ranges between 31% and 6%.3,4
Among the conclusions, it should be pointed out that our experience is similar to that of other centers and the most representative of those published in Spain. We advocate the technique of choice for most authors, with division and reimplantation, which can be performed with or without ECC, depending on the status of the patient. The mortality, the rate of which was comparable to that of other centers, was due to respiratory complications. Our approach to the airway was conservative. We have not performed tracheal surgery in mild or moderate tracheal stenoses or in infants under three months of age because of the high mortality rate in this age group. We have intervened in cases of severe obstruction when the symptomatology did not improve, using endoscopic techniques. In five patients, older infants, it was not necessary to employ any therapeutic measures involving the airway. In two patients, very young infants with very severe stenoses who, in addition, required more prolonged mechanical ventilation, endoscopic techniques were carried out (balloon dilatations, and one also underwent stent placement) that enabled a favorable management of one case. Obviously, the number of patients and length of follow-up lead us to be prudent when it comes to formulating definitive conclusions.
Conflicts of interestNone declared.
Received 31 March 2010
Accepted 3 June 2010
Corresponding autor: Servicio de Cardiología, Hospital Sant Joan de Dèu, P.o Sant Joan de Dèu 2, 08950 Esplugues, Barcelona, Spain. jcarretero@hsjdbcn.org