Aortic regurgitation usually occurs during diastole, although in certain situations, such as atrial fibrillation or extrasystoles, systolic aortic regurgitation (SAR) has been reported. This phenomenon is a reflection of mechanical failure of the ventricle and arises because the left ventricle is unable to generate the necessary pressure to open the aortic valve, leading to a systolic gradient between the aorta and the left ventricle. In fact, SAR has been associated with the presence of heart failure (HF).1 The objective of this study was to assess the prevalence of SAR in an unselected outpatient population and determine the relationship between SAR and HF.
In this prospective observational study lasting 15 months (January 2012 through March 2013), data were collected from all outpatients referred to our echocardiography laboratory, where the same operator recorded all echocardiograms. The presence of HF was assessed according to the current clinical practice guidelines.2 Patients with aortic regurgitation of any severity and atrial fibrillation or extrasystoles underwent a 60-s continuous-wave Doppler assessment of aortic regurgitation in the 5-chamber apical view. In the echocardiography study, SAR was defined as the presence of holosystolic flow from the aorta to the left ventricular outflow tract. Admissions to hospital and deaths due to HF were recorded for patients with SAR.
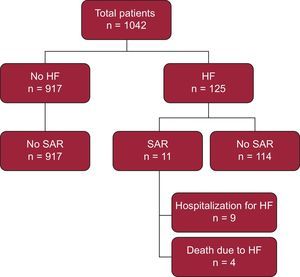
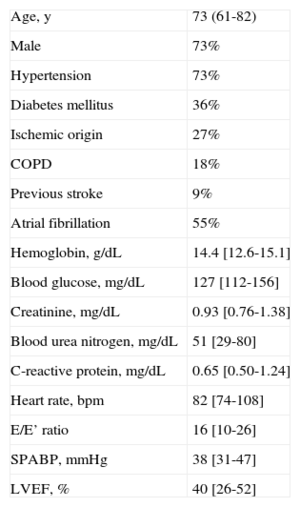
Overall, 1042 patients were included (Figure). The prevalence of SAR was 1% (n = 11) and the underlying cause was ventricular extrasystoles in 6 patients and atrial fibrillation in 5. The heart rate of patients with SAR was 82 (74-108) bpm and the RR interval prior to an event was 440 (377-547) ms. Severe ventricular arrhythmias were not observed. The prevalence of HF was 12%. All patients with SAR had HF. Thus, in the subpopulation of patients with HF, SAR was found in 9%. As shown in Table, the age of the patients with SAR was 73 (61-82) years, the left ventricular ejection fraction (LVEF) was 40% (26%-52%), and there was no significant decline in renal function or anemia. During follow-up (350 [SD, 184] days), 9 of the 11 patients with SAR were admitted to hospital for HF, an event that occurred soon after diagnosis of SAR (76 [SD, 99] days). In addition, 4 out of 11 patients with SAR died of HF.
Baseline Characteristics of Patients With Systolic Aortic Regurgitation (n = 11)
| Age, y | 73 (61-82) |
| Male | 73% |
| Hypertension | 73% |
| Diabetes mellitus | 36% |
| Ischemic origin | 27% |
| COPD | 18% |
| Previous stroke | 9% |
| Atrial fibrillation | 55% |
| Hemoglobin, g/dL | 14.4 [12.6-15.1] |
| Blood glucose, mg/dL | 127 [112-156] |
| Creatinine, mg/dL | 0.93 [0.76-1.38] |
| Blood urea nitrogen, mg/dL | 51 [29-80] |
| C-reactive protein, mg/dL | 0.65 [0.50-1.24] |
| Heart rate, bpm | 82 [74-108] |
| E/E’ ratio | 16 [10-26] |
| SPABP, mmHg | 38 [31-47] |
| LVEF, % | 40 [26-52] |
COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; SPABP, systolic pulmonary artery blood pressure.
Qualitative variables are expressed as percentages and quantitative variables as mean (range) or median [interquartile range].
The prevalence of SAR, although low, is not negligible, and increases notably among patients with HF (P < .001). SAR is a reflection of ventricular mechanical failure and has been associated with HF.1 In addition, our findings indicate a close relationship between SAR and HF, as all patients with SAR had HF. It should be mentioned that LVEF in patients with SAR was 40% (26%-52%) and that SAR was observed over the whole spectrum of HF (from decreased LVEF to preserved LVEF). In fact, 46% of our patients with SAR had LVEF > 50%. The RR interval prior to SAR was 440 (377-547) ms, and short diastoles were suspected to be the trigger. However, an early heartbeat is not in itself responsible for SAR, so other factors such as distensibility and myocardial contractility probably contribute.
Our analysis shows poor clinical outcomes in patients with SAR, as mortality and hospitalization rates are high for HF. In fact, whereas mortality due to HF is a complex situation that often cannot be addressed, hospitalization due to HF is closely related to disease progression and there is the potential for change through preventive strategies and multifactorial management.3 If these findings are confirmed, patients with SAR might benefit from preventive measures.
The study is subject to limitations. First, this is a single-center study in which patients with HF but without SAR were not analyzed. Thus, we do not know the differences in baseline characteristics between the 2 subpopulations and the real association between SAR and worse clinical outcome. Likewise, the possible value added to other echocardiographic parameters such as pulmonary hypertension and E/E’ ratio could not be derived. The limited number of patients with SAR, due to its low prevalence, prevents us from drawing firm conclusions and could limit their usefulness. However, the characteristics of our patients were similar to those of other series with event rates lower than those observed in our population of patients with SAR (death due to HF of 17% vs 36%; hospitalization/death due to HF, 45% vs 82%).4
New studies are needed to assess the association between SAR and HF and its additional prognostic value. We have no information on the prevalence of SAR in acute HF or indeed whether its resolution may be beneficial in these patients. In short, this phenomenon appears to be associated with worse clinical outcomes in patients with HF. Given the ease of diagnosis and lack of added costs, such assessments should be included in the echocardiographic study of our patients with HF who present with some degree of aortic regurgitation and atrial fibrillation or extrasystole. The clinical cardiologist should bear this in mind in this subgroup of patients, given the apparent implications.