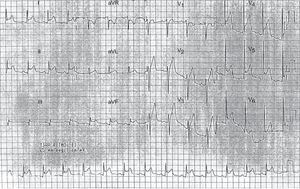
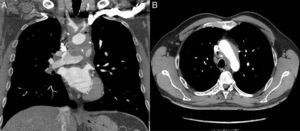
A 39-year-old man with no past medical history of note presented to the emergency department with a 3-day history of chest pain, worse on inspiration and lying down, along with systemic symptoms (low-grade fever, weakness, and malaise) for 2 months. There was no pericardial rub, and there were no murmurs or signs of heart failure. Observations were within normal limits. Electrocardiogram showed diffuse concave ST elevation and PR depression compatible with pericarditis (Figure 1), and blood tests showed a high C-reactive protein and erythrocyte sedimentation rate (12.3 mg/dL and 98 mm/h), with no increase in markers of myocardial injury. Chest X-ray and transthoracic echocardiography were normal, and the patient was discharged with ibuprofen (600 mg/8 h) and colchicine (1 g/24 h). Two weeks later, the patient was seen in the cardiology clinic and reported no improvement. On examination, a left carotid bruit and a weak carotid pulse were detected. Therefore, the patient was sent to the emergency department, where computed tomography angiography was performed. This showed wall thickening of the aortic arch and supra-aortic trunks with partial obliteration of the lumen, particularly in the left common carotid artery (Figure 2), compatible with Takayasu arteritis (TA), and mild pericardial thickening without effusion. The patient was admitted to rheumatology, where he received corticosteroids, with a good response.
Systemic rheumatologic diseases are autoimmune disorders that affect multiple organs. These diseases can affect the heart, which clouds the diagnosis. Some patients require aggressive immunosuppressive therapy and revascularization and/or valve replacement.1,2 Pericardial involvement in these diseases generally reflects the degree of disease activity. Some 5% to 15% of patients with acute or recurrent pericarditis have a systemic autoimmune disease,3 with pericarditis being the most common cardiac manifestation of systemic lupus erythematosus (20%-50% of patients and more than 60% at postmortem) and rheumatoid arthritis (40%). In scleroderma, pericarditis is usually asymptomatic (although found in 70% at postmortem), while in ankylosing spondylitis, pericardial involvement is rare. The primary systemic vasculitides affect the heart in 10% of cases (60% in TA and in Churg-Strauss); however, pericardial involvement is uncommon, and even less so as the first manifestation of the disease.1,2
Takayasu arteritis is a chronic inflammatory granulomatous vasculitis that causes stenosis of the aorta and its major branches (carotid, subclavian, pulmonary, and coronary arteries). It mostly affects young women (women:men, 6-10:1) and is very uncommon (2.6/million population in the United States and 1.26/million population in northern Europe, with a higher prevalence in Asia).2,4 Cardiac involvement is one of the main causes of morbidity and mortality in TA and can present as pericarditis, myocarditis, coronary arteritis with myocardial ischaemia, valve disease (aortic regurgitation is the most common, followed by mitral regurgitation) or intracardiac thrombus.1,5 There are few reported cases of pericarditis secondary to TA. These patients usually present with signs and symptoms of pericarditis, accompanied by systemic symptoms (weakness, fever, malaise), raised acute phase reactants and signs of the TA itself, such as asymmetrical pulses (“pulseless disease”), carotidynia, unexplained hypertension in young patients, arterial bruits, intermittent claudication, or angina.2–4 Angiography is the standard diagnostic test but is being replaced by other less invasive methods such as computed tomography angiography and magnetic resonance angiography, which allow assessment of wall thickness and avoid false negatives in the early phase of arteriography. The preferred method for follow-up is magnetic resonance angiography, due to the lack of ionizing radiation.4,6 Doppler echocardiography can detect reduced vessel diameter, prestenotic dilatations, and wall thickening, although it has limitations (gas interposition, obesity, etc).6 On positron emission computed tomography, which is less available, inflamed vessel walls can be seen as areas of enhancement.4
The mainstay of treatment for acute pericarditis is nonsteroidal anti-inflammatory drugs and colchicine (to prevent recurrence), while corticosteroids are the second-line treatment, if the disease fails to respond.3 If corticosteroids are used, the dose should be low (prednisolone 0.2 to 0.5mg/kg/day or equivalent). However, in TA, corticosteroids are the treatment of choice, and high doses are required (1 mg/kg/d), with a good response in most patients, but relapses are frequent when the dose is reduced.4 Good results can also be achieved with methotrexate and biological treatments (antitumour necrosis factor antibodies [infliximab, etanercept, adalimumab], anti-CD20 monoclonal antibodies [rituximab], interleukin 6 receptor antagonists [tocilizumab], and the CTLA-4-Ig fusion protein [abatacept]). In cases of intolerance or contraindication to these therapies, the alternatives are cyclophosphamide, azathioprine, and mycophenolate mofetil.4
In this patient with acute pericarditis as the first symptom of TA, in whom some key features initially passed unnoticed (systemic symptoms, raised acute phase reactants, asymmetrical pulses), the poor response to treatment and a further detailed examination led to the diagnosis of underlying TA. In cases of rheumatological disease with initial symptoms related to the heart, the cardiologist may be the first physician to see the patient; therefore, familiarity with these symptoms is essential to achieve an early diagnosis. In addition, some of the treatments used in these types of disease can have different effects on vascular risk (a negative effect in the case of corticosteroids)5; therefore the cardiologist also has an important role at follow-up.