In the face of declining mortality due to ischemic heart disease, patients with coronary artery disease currently have a higher burden of comorbidity, more complex coronary anatomy, and a higher prevalence of severe calcification.1 Chronic total occlusions (CTO) are commonly encountered among patients with coronary artery disease, as they are detected in one-fifth of subjects undergoing coronary angiography.2 The composition of the occlusive atherosclerotic plaque varies according to the duration of the occlusion and the clinical setting (ie, in-stent CTO, or patients with a prior coronary artery bypass graft).3 However, most CTO are extensively calcified, with calcifications taking up to 30% of plaque volume.4 Notably, the presence of severe calcifications is associated with a lower procedural success rate and worse long-term clinical outcomes after percutaneous coronary intervention (PCI) in all-comers.4 The adverse effects of coronary artery calcification include impaired balloon and stent deliverability, as well as inadequate stent expansion, which leads to suboptimal PCI result. Dedicated plaque-modifying devices (PMDs), including specialty balloons, atherectomy and intravascular lithotripsy, have been developed in the last 3 decades to modify calcium, increase lesion compliance and, ultimately, improve acute and long-term outcomes. Because more than 50% of CTOs are severely calcified,5 the use of PMDs in this setting is particularly appealing. The use of PMD in CTO PCI, however, has been the subject of surprisingly few studies.6–8
In a recent article published in Revista Española de Cardiología, Delgado-Arana et al.9 present an interesting analysis of the contemporary use of PMDs in the setting of CTO PCI. The analysis included 2235 patients treated with CTO PCI from 2015 to 2020 in 17 centers in Spain. Wire crossing was successful in 85% of patients, and procedural success was achieved in 75%. PMDs were used in 7% of patients, with only 1% requiring more than 1 device. The most commonly used PMDs were rotational atherectomy (in 51% of patients requiring a PMD) and cutting/scoring balloons (in 42%). As expected, the use of PMDs was associated with older patient age and higher SYNTAX score. On univariate analysis, patients in the PMD group showed higher rates of procedural success.
There seemed to be 2 different patterns in PMD use in this study: while higher-volume centers more frequently used an atherectomy-based approach (mostly rotational atherectomy), lower-volume institutions relied more on balloon-based technology, which can be interpreted in the context of the more technically demanding nature of atherectomy procedures. Patients in the PMD group more frequently had in-hospital myocardial infarction (0.5% vs 2.2% in non-PMD vs PMD group, respectively; P=.047), but the incidence of other adverse outcomes did not differ at the 24-month follow-up.
The authors are to be congratulated for their effort in addressing this mostly unanswered clinical question, and for the timely analysis of calcific plaque modification, which is a matter of intense debate after recent innovations in the field. Furthermore, their article provides an updated picture of CTO PCI practice in southern Europe.
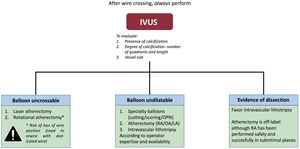
However, some limitations must also be acknowledged. First, very few patients were enrolled by each center over the 6-year span of the study (∼22 procedures/center/y, but with wide variation across centers), which hints at nonconsecutive reporting or alternatively very low CTO PCI volume on the one hand, and a marked heterogeneity in clinical practice on the other. Moreover, intravascular imaging appears to be underused in this cohort, with just 14% of patients undergoing intravascular ultrasound, which compares with ∼40% in large international registries.10 Intravascular imaging has proved to be superior to angiography in identifying calcification. Indeed, Delgado-Arana et al.9 report severe calcification in only 65.7% of participants requiring PMDs, which indirectly seems to confirm an issue of calcification underdiagnosis. The small proportion of intravascular imaging use and the lack of a core laboratory angiographic/imaging analysis surely limits further insights on this issue. These authors strongly advocate for systematic use of intravascular ultrasound after CTO crossing, which allows adequate assessment of the calcification burden and can be used to inform subsequent lesion modification strategies and optimize stent expansion (figure 1). Of note, such an approach could streamline the selection and use of PMD, with a possible reduction in procedural times due to optimal device choice.
Choice of plaque modification devices in chronic total occlusion percutaneous coronary intervention. We recommend performing intravascular ultrasound in all patients after chronic total occlusion crossing and before lesion preparation to evaluate the degree of calcification, and inform the indication and selection of plaque modification devices. IVL, intravascular lithotripsy; IVUS, intravascular ultrasound; LA, laser atherectomy; OA, orbital atherectomy; RA, rotational atherectomy.
Second, the average J-CTO score in this study was markedly lower than that in previously reported large-scale registries, with Delgado-Arana et al.9 reporting an average J-CTO of 2.2±1.1 among patients requiring PMD and 1.8±1.1 among non-PMD patients, while in the Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS-CTO) registry these figures were 3.0±1.2 and 2.4±1.3, respectively.11 The proportion of patients with prior coronary artery bypass graft surgery was also lower than previously reported: 6.6% in the present work, which compares with figures ranging between 20% and 32% in the literature.8,11 Of note, both these parameters are associated with lower procedural success and more severe CTO calcification. In addition,> 80% of procedures were carried out with an antegrade approach, while most international registries report figures of ∼50% to 60% for antegrade wiring.11 Altogether, these data point to a potential selection bias to overall lower patient risk and procedural complexity, potentially driving the excellent short- and long-term outcomes in this cohort (target lesion revascularization 3.4%–but only 0.7% in the PMD group–on follow-up).9
Moreover, the authors do not report the proportion of patients lost to follow-up. The rates of adverse outcomes appear to be markedly lower than those reported in other studies. Finally, some key, standardized outcomes recommended by the CTO Academic Research Consortium,12 including target lesion failure, are not reported, which hampers comparisons with the literature.
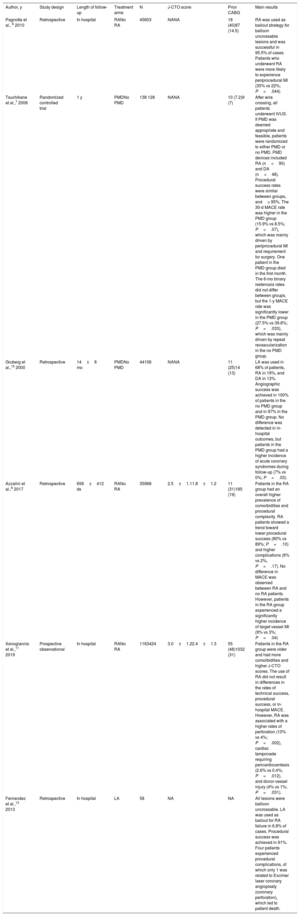
Of interest, most previously published work on calcification management strategies in CTO PCI have focused mainly on rotational atherectomy. In the largest registries to date, Azzalini et al.8 and Xenogiannis et al.11 reported the use of rotational atherectomy in 3.5% and 3.2% of cases, respectively, which is in line with the results reported by Delgado-Arana et al.9 Very few studies have reported the use of laser atherectomy in CTO PCI, which appears to be an attractive option in balloon-uncrossable lesions, especially when rotational atherectomy has also failed, as it allowed for a procedural success exceeding 90% and low complication rates in one series.13 Given the relatively recent introduction of intravascular lithotripsy, only case reports and case series are available on this device and solid conclusions cannot be drawn on its efficacy and safety in the CTO PCI setting.14 Of note, intravascular lithotripsy appears to be effective and remarkably safe, although the unfavorable crossing profile of intravascular lithotripsy balloons may require combination with other PMD approaches to allow their use. Table 1 summarizes the main studies that have analyzed the use of several PMDs in the setting of CTO PCI.
Most relevant studies on the use of plaque modifying devices in the setting of chronic total occlusion percutaneous coronary intervention
| Author, y | Study design | Length of follow-up | Treatment arms | N | J-CTO score | Prior CABG | Main results |
|---|---|---|---|---|---|---|---|
| Pagnotta et al., 6 2010 | Retrospective | In hospital | RANo RA | 45603 | NANA | 18 (40)87 (14.5) | RA was used as bailout strategy for balloon uncrossable lesions and was successful in 95.5% of cases. Patients who underwent RA were more likely to experience periprocedural MI (35% vs 22%; P=.044) |
| Tsuchikane et al.,7 2006 | Randomized controlled trial | 1 y | PMDNo PMD | 138 128 | NANA | 10 (7.2)9 (7) | After wire crossing, all patients underwent IVUS. If PMD was deemed appropriate and feasible, patients were randomized to either PMD or no PMD. PMD devices included RA (n=90) and DA (n=48). Procedural success rates were similar between groups, and> 95%. The 30-d MACE rate was higher in the PMD group (15.9% vs 8.5%; P=.07), which was mainly driven by periprocedural MI and requirement for surgery. One patient in the PMD group died in the first month. The 6-mo binary restenosis rates did not differ between groups, but the 1-y MACE rate was significantly lower in the PMD group (27.5% vs 39.8%; P=.033), which was mainly driven by repeat revascularization in the no PMD group. |
| Gruberg et al.,15 2000 | Retrospective | 14±8 mo | PMDNo PMD | 44106 | NANA | 11 (25)14 (13) | LA was used in 68% of patients, RA in 19%, and DA in 13%. Angiographic success was achieved in 100% of patients in the no PMD group and in 97% in the PMD group. No difference was detected in in-hospital outcomes, but patients in the PMD group had a higher incidence of acute coronary syndromes during follow-up (7% vs 0%; P=.03). |
| Azzalini et al.,8 2017 | Retrospective | 658±412 ds | RANo RA | 35968 | 2.5±1.11.8±1.2 | 11 (31)185 (19) | Patients in the RA group had an overall higher prevalence of comorbidities and procedural complexity. RA patients showed a trend toward lower procedural success (80% vs 89%; P=.10) and higher complications (6% vs 2%, P=.17). No difference in MACE was observed between RA and no RA patients. However, patients in the RA group experienced a significantly higher incidence of target-vessel MI (9% vs 3%; P=.04) |
| Xenogiannis et al.,11 2019 | Prospective observational | In hospital | RANo RA | 1163424 | 3.0±1.22.4±1.3 | 55 (48)1032 (31) | Patients in the RA group were older and had more comorbidities and higher J-CTO scores. The use of RA did not result in differences in the rates of technical success, procedural success, or in-hospital MACE. However, RA was associated with a higher rates of perforation (10% vs 4%; P=.002), cardiac tamponade requiring pericardiocentesis (2.6% vs 0.4%; P=.012), and donor-vessel injury (4% vs 1%; P=.031). |
| Fernandez et al.,13 2013 | Retrospective | In hospital | LA | 58 | NA | NA | All lesions were balloon uncrossable. LA was used as bailout for RA failure in 6.8% of cases. Procedural success was achieved in 91%. Four patients experienced procedural complications, of which only 1 was related to Excimer laser coronary angioplasty (coronary perforation), which led to patient death. |
CABG, coronary artery bypass graft; DA, directional atherectomy; IVUS, intravascular ultrasound; J-CTO, Japan chronic total occlusion; LA, laser atherectomy; MACE, major adverse cardiovascular events; MI, myocardial infarction; NA, not available; PMD, plaque modification device; RA, rotational atherectomy.
The results are reported as No. (%) or mean±standard deviation.
Despite the aforementioned limitations, the study by Delgado-Arana et al.9 offers a contemporary snapshot of CTO PCI practice in Spain, and provides reassurance on the safety and effectiveness of PMDs in the setting of these complex interventions. Further studies are needed to further refine our understanding of the role of PMDs in the setting of CTO PCI, and to ultimately guide the selection of the most appropriate device for each patient. Our algorithm for the optimal selection and use of PMD is presented in figure 1.
FUNDINGNone.
CONFLICTS OF INTERESTF. Moroni has no conflicts of interest. L. Azzalini declares honoraria from Teleflex, Abiomed, Asahi Intecc, Philips, Abbott Vascular, and Cardiovascular System, Inc.