There is little information on the incidence and predictors of infarction, stroke, or cardiovascular death after acute coronary syndrome. We investigated these aspects and developed tools for predicting these events according to the time of their occurrence.
MethodsA retrospective study was conducted of 4858 patients who survived an acute coronary event. We analyzed the incidence and predictors of acute myocardial infarction, stroke, or cardiovascular death during the first year (n=4858) vs successive years (n=4345 patients free of composite events during the first year).
ResultsThere were 329 events in the first year (cumulative incidence function: 7.3% person-years) and 616 in successive years (21.5% person-years; follow-up 4.9±2.4 years). The risk of events during the first year per tertile was 2.5% person-years in the low-risk tertile (< 3 points), 4.8% person-years in the intermediate-risk tertile (3-6 points), and 15.5% person-years in the high-risk tertile (> 6 points) (P<.001). The risk of events in the cohort that had a combined event in successive years increased from 10.7% person-years in the low-risk tertile (< 3 points) to 40.3% person-years in the high-risk tertile (> 6 points) (P<.001). The 2 scales showed the following predictive indexes: C statistic, 0.74 and 0.69, respectively; P (Hosmer-Lemeshow test)≥0.44
ConclusionThe risk of recurrence of cardiovascular events remains high after acute coronary syndrome. The level of risk can be easily quantified with acceptable predictive ability.
Keywords
The incidence and prognosis of coronary disease after acute coronary syndrome (ACS) in the short- and medium-term are among the most widely studied areas in cardiology.1–8 However, there is little information on the incidence and determinants of new adverse cardiovascular events during the long-term clinical course.9–11 Moreover, the limited information available has been obtained from studies of populations of ACS patients in whom coronary revascularization was little used9–11 even in the most contemporary series.9
On the other hand, the effect of many factors associated with the development of cardiovascular events after an ACS is often not considered according to whether the follow-up is short-to-medium term or long-term. Characterizing the clinical course after ACS in relation to major adverse cardiovascular events and identifying the precursors of such events could help to individualize clinical follow-up, assess the effectiveness of treatments, and optimize resource allocation. These aspects gain further relevance regarding forecasts of the substantial burden of morbidity and costs of ischemic heart disease12–14 and the importance of individual risk stratification in chronic disease management models.
In a cohort of ACS patients with a high rate of coronary revascularization, we studied the risk of acute myocardial infarction (AMI), stroke, or cardiovascular death during the first year of follow-up (adjusting for noncardiovascular mortality as a competing event) and compared it with the risk of these events in successive years. We also attempted to facilitate estimation of the risk of the 3 events by creating 2 intuitive clinical tools.
METHODSStudy PopulationA retrospective study was conducted based on the CardioCHUS registry, which included all consecutive patients with a primary and final diagnosis of ACS (n=5203) admitted to the Cardiology Service of the Hospital Clínico Universitario de Santiago de Compostela (Spain) from December 2003 to September 2012. The study excluded patients who died in hospital (n=291) or who were lost to follow-up (n=54). The final cohort consisted of 4858 patients.
The study was conducted in accordance with the principles of the Declaration of Helsinki.
Objectives and Follow-upThe primary objective of the study was to determine the incidence and predictors of the composite of AMI, stroke, or cardiovascular death after ACS during the first year vs successive years in the subgroup of patients free of composite events during the first year.
Nonfatal AMI during follow-up was defined as the first hospitalization for AMI (according to compatible symptoms, elevated troponin I levels above normal laboratory limits, with or without electrocardiographic changes). A first nonfatal ischemic stroke was defined as a definitive diagnosis by the attending neurologist of a new persistent neurological deficit supported by the results of at least 1 imaging test.
Cause of death was classified according to the method previously used by our group.15 Cardiovascular and noncardiovascular death were defined as mortality due to sudden death, refractory heart failure, ACS, acute aortic syndrome, pulmonary, systemic, or cerebral thrombosis, or renal vascular disease (kidney failure in the absence of glomerulopathy or other parenchymal abnormalities). Other causes of death were considered to be of noncardiovascular origin. Two cardiologists (N. Bouzas-Cruz and E. Abu-Assi) assigned the cause of death. A third cardiologist (J.M. García Acuña) was consulted if there was a difference of opinion. In the absence of information or when there was no consensus on the cause, death was classified as “unknown or unclassifiable cause of death”.
Events during follow-up were identified using electronic medical records for the autonomous community of Galicia (IANUS software). Between January and March 2014, all medical care and hospital records were reviewed; telephone contact was used in specific cases.
Statistical AnalysisQuantitative variables are expressed as mean±standard deviation and categorical variables as percentages. Qualitative variables were compared using the chi-square test or Fisher's exact test and quantitative variables were compared using the Student t test.
The adjusted composite event rate was estimated using the cumulative incidence function (CIF) during the 2 study periods: a) during the first year, and b) during successive years in patients free of composite events during the first year.
Because of competing risks between noncardiovascular death and AMI, stroke, or cardiovascular death, we used the competing risks analysis developed by Fine and Gray16 to estimate the incidence of these events and identify their predictors.
Variables shown to be associated with the event of interest by bivariate analysis using a P value of < .10 were included in the multivariable prediction model of the risk of a composite event during the first year. The variables sex, year of admission, and implantable cardioverter defibrillator were forced into the model. The model included age, sex, smoking, hypertension, diabetes mellitus, peripheral vascular disease, previous stroke or transient ischemic attack, previous ischemic heart disease (previous AMI or coronary revascularization), chronic obstructive pulmonary disease, history of atrial fibrillation (prior to or during admission), history of heart failure (prior to or during admission), heart rate on admission, non—ST-segment elevation AMI, serum creatinine and hemoglobin at admission, multivessel disease (at least 2 main coronary arteries with stenosis ≥ 70% or ≥ 50% if the left main coronary artery was involved), absence of coronary revascularization during the index event, failed percutaneous coronary intervention (final TIMI [Thrombolysis In Myocardial Infarction] flow < 3 or residual stenosis > 30%), moderate or severe in-hospital bleeding according to the TIMI scale,17 implantable cardioverter defibrillator, hospital stay (loge), and year of admission.
Variables shown to be associated in the bivariate analysis using a P value of < .1 were included in the multivariable prediction model of the risk of a composite event in successive years: age, smoking, hypertension, diabetes mellitus, peripheral vascular disease, previous stroke or transient ischemic attack, previous ischemic heart disease, chronic obstructive pulmonary disease, history of atrial fibrillation, history of heart failure, heart rate on admission, non—ST-segment elevation AMI, serum creatinine and hemoglobin at admission, significant multivessel disease, absence of coronary revascularization during the index event, moderate or severe in-hospital bleeding according to the TIMI scale,17 implantable cardioverter defibrillator, hospital stay (loge), and previous cancer. The variables sex and year of admission were forced into the model.
Fractional polynomials were used to determine the functional form of the quantitative variables (log-linear relationship).18 The proportional hazards assumption was tested by studying interactions between the covariates in the models over time: the absence of statistical significance (P>.05) showed that the proportional hazards assumption was not violated.
The bootstrap method (1000 iterations) was used to calculate and estimate statistical significance and sub-hazard ratios and their 95% confidence intervals (95%CI).
We developed a risk score based on that of Sullivan et al19 in order to make it easier for clinicians to estimate risk obtained from the multivariable models. The risk score was proportionally assigned to the coefficients of the variables with P<.05 in the final multivariable models. Scores were rounded to 0.5 or the nearest integer. Subsequently, we established the risk scales according to the risk scores obtained and calculated the risk probability corresponding to these scores.
Expected vs observed events and the Hosmer-Lemeshow test were used to calibrate the risk scale and assess its ability to assign patients to risk groups. The C statistic was used to assess the discriminative ability of the risk score.
Left ventricular ejection fraction (LVEF) (not quantified in 467 patients) was not taken into account when deriving the predictive scales, thus we assessed discriminative ability by subgroups with LVEF < 35%, 35-45%, > 45%, or unavailable LVEF.
All statistical analyses were performed using the R and STATA 13 statistical software packages.
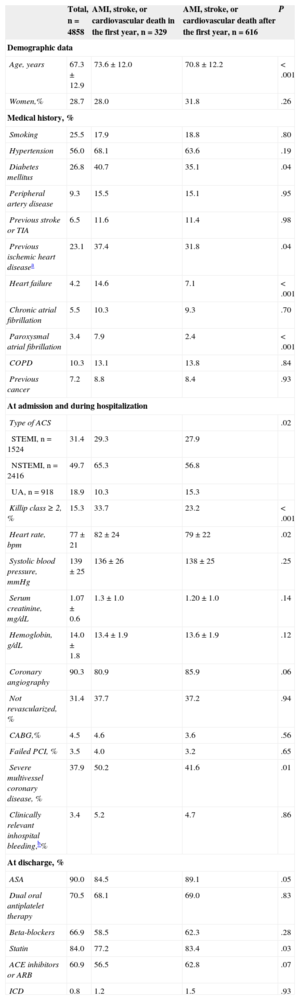
RESULTSTable 1 shows the baseline characteristics of the 4858 patients (mean age=67.3 years; 28.7% women). Of these patients, 90.3% underwent coronary angiography and 68.6% were revascularized.
Baseline Characteristics of the Total Study Population and Stratified by Subgroups According to the Time of Acute Myocardial Infarction, Stroke, or Cardiovascular Death
| Total, n=4858 | AMI, stroke, or cardiovascular death in the first year, n=329 | AMI, stroke, or cardiovascular death after the first year, n=616 | P | |
|---|---|---|---|---|
| Demographic data | ||||
| Age, years | 67.3±12.9 | 73.6±12.0 | 70.8±12.2 | < .001 |
| Women,% | 28.7 | 28.0 | 31.8 | .26 |
| Medical history, % | ||||
| Smoking | 25.5 | 17.9 | 18.8 | .80 |
| Hypertension | 56.0 | 68.1 | 63.6 | .19 |
| Diabetes mellitus | 26.8 | 40.7 | 35.1 | .04 |
| Peripheral artery disease | 9.3 | 15.5 | 15.1 | .95 |
| Previous stroke or TIA | 6.5 | 11.6 | 11.4 | .98 |
| Previous ischemic heart diseasea | 23.1 | 37.4 | 31.8 | .04 |
| Heart failure | 4.2 | 14.6 | 7.1 | < .001 |
| Chronic atrial fibrillation | 5.5 | 10.3 | 9.3 | .70 |
| Paroxysmal atrial fibrillation | 3.4 | 7.9 | 2.4 | < .001 |
| COPD | 10.3 | 13.1 | 13.8 | .84 |
| Previous cancer | 7.2 | 8.8 | 8.4 | .93 |
| At admission and during hospitalization | ||||
| Type of ACS | .02 | |||
| STEMI, n=1524 | 31.4 | 29.3 | 27.9 | |
| NSTEMI, n=2416 | 49.7 | 65.3 | 56.8 | |
| UA, n=918 | 18.9 | 10.3 | 15.3 | |
| Killip class ≥ 2, % | 15.3 | 33.7 | 23.2 | < .001 |
| Heart rate, bpm | 77±21 | 82±24 | 79±22 | .02 |
| Systolic blood pressure, mmHg | 139±25 | 136±26 | 138±25 | .25 |
| Serum creatinine, mg/dL | 1.07±0.6 | 1.3±1.0 | 1.20±1.0 | .14 |
| Hemoglobin, g/dL | 14.0±1.8 | 13.4±1.9 | 13.6±1.9 | .12 |
| Coronary angiography | 90.3 | 80.9 | 85.9 | .06 |
| Not revascularized, % | 31.4 | 37.7 | 37.2 | .94 |
| CABG,% | 4.5 | 4.6 | 3.6 | .56 |
| Failed PCI, % | 3.5 | 4.0 | 3.2 | .65 |
| Severe multivessel coronary disease, % | 37.9 | 50.2 | 41.6 | .01 |
| Clinically relevant inhospital bleeding,b% | 3.4 | 5.2 | 4.7 | .86 |
| At discharge, % | ||||
| ASA | 90.0 | 84.5 | 89.1 | .05 |
| Dual oral antiplatelet therapy | 70.5 | 68.1 | 69.0 | .83 |
| Beta-blockers | 66.9 | 58.5 | 62.3 | .28 |
| Statin | 84.0 | 77.2 | 83.4 | .03 |
| ACE inhibitors or ARB | 60.9 | 56.5 | 62.8 | .07 |
| ICD | 0.8 | 1.2 | 1.5 | .93 |
ACE, angiotensin-converting enzyme; ACS, acute coronary syndrome; AMI, acute myocardial infarction; ARB, angiotensin receptor blockers; ASA: acetylsalicylic acid; CABG, coronary artery bypass surgery; COPD, chronic obstructive pulmonary disease; ICD, implantable cardioverter defibrillator; NSTEMI, non—ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TIA, transient ischemic attack; UA, unstable angina.
History of acute myocardial infarction or previous percutaneous or surgical coronary revascularization.
Moderate or severe in-hospital bleeding according to the Thrombolysis In Myocardial Infarction scale.17
During 4.8±2.6 years of follow-up, there were 945 AMI, strokes, or cardiovascular deaths.
During the first year, 329 patients had a composite event (CIF = 7.3 [95%CI, 6.5-8.1]/100 person-years): The 329 events comprised 185 AMI, 40 strokes, and 104 cardiovascular deaths.
During the first year, 184 patients died of nonvascular causes. Thus, during the second follow-up period, the second cohort comprised the 4345 patients who survived the first year and remained event-free.
These patients were followed-up for 4.9±2.4 years. In total, 616 had a composite event (21.5 [95%CI, 18.3-24.7]/100 person-years). In the second cohort, the CIF at 1, 2, 3, 4, and 5 years of follow-up was 3.5, 6.0, 8.6, 10.1, and 13.2 events/100 person-years, respectively.
The 616 events during the second follow-up period comprised 274 AMI, 133 strokes, and 209 cardiovascular deaths.
Some characteristics were more frequent among patients who had a composite event during the first year: the patients were nearly 3 years older (73.6 years vs 70.8 years; P<.001), had a significantly higher prevalence of previous ischemic heart disease, diabetes mellitus, heart failure, and atrial fibrillation (Table 1), and more frequently had non—ST-segment elevation AMI and poor kidney function at admission. They were also less frequently prescribed acetylsalicylic acid, statins, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers.
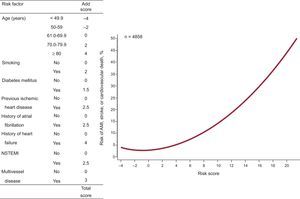
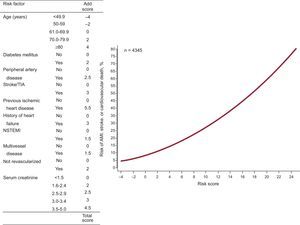
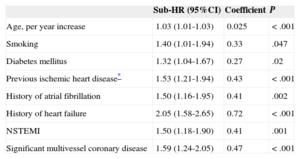
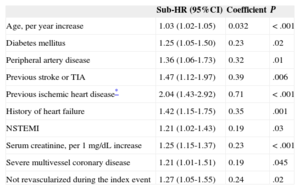
Eight independent predictors of composite events were identified during the first year and 10 were identified during the successive years (Tables 2 and 3) (the effect of all the prognostic factors that composed the multivariable models is shown in ).
Effect of Independent Predictors in the Final Multivariable Model for the Prediction of Acute Myocardial Infarction, Stroke, or Cardiovascular Death During the First Year of Follow-up
| Sub-HR (95%CI) | Coefficient | P | |
|---|---|---|---|
| Age, per year increase | 1.03 (1.01-1.03) | 0.025 | < .001 |
| Smoking | 1.40 (1.01-1.94) | 0.33 | .047 |
| Diabetes mellitus | 1.32 (1.04-1.67) | 0.27 | .02 |
| Previous ischemic heart disease* | 1.53 (1.21-1.94) | 0.43 | < .001 |
| History of atrial fibrillation | 1.50 (1.16-1.95) | 0.41 | .002 |
| History of heart failure | 2.05 (1.58-2.65) | 0.72 | < .001 |
| NSTEMI | 1.50 (1.18-1.90) | 0.41 | .001 |
| Significant multivessel coronary disease | 1.59 (1.24-2.05) | 0.47 | < .001 |
95%CI, 95% confidence interval; HR, hazard ratio; NSTEMI, non—ST-segment elevation myocardial infarction.
Effect of Independent Predictors in the Final Multivariable Model for the Prediction of Myocardial Infarction, Stroke, or Cardiovascular Death After the First Year
| Sub-HR (95%CI) | Coefficient | P | |
|---|---|---|---|
| Age, per year increase | 1.03 (1.02-1.05) | 0.032 | < .001 |
| Diabetes mellitus | 1.25 (1.05-1.50) | 0.23 | .02 |
| Peripheral artery disease | 1.36 (1.06-1.73) | 0.32 | .01 |
| Previous stroke or TIA | 1.47 (1.12-1.97) | 0.39 | .006 |
| Previous ischemic heart disease* | 2.04 (1.43-2.92) | 0.71 | < .001 |
| History of heart failure | 1.42 (1.15-1.75) | 0.35 | .001 |
| NSTEMI | 1.21 (1.02-1.43) | 0.19 | .03 |
| Serum creatinine, per 1 mg/dL increase | 1.25 (1.15-1.37) | 0.23 | < .001 |
| Severe multivessel coronary disease | 1.21 (1.01-1.51) | 0.19 | .045 |
| Not revascularized during the index event | 1.27 (1.05-1.55) | 0.24 | .02 |
95%CI, 95% confidence interval; HR, hazard ratio; NSTEMI, non—ST-segment elevation myocardial infarction.
These predictors were used to design 2 intuitive quantitative tools to estimate the composite event risk during the first year (Figure 1) and during successive years (Figure 2).
The distribution of the risk scores for AMI, stroke, or cardiovascular death during or after the first year is shown in . During the first year, the risk score values were -4 to 22, whereas in successive years they were –4 to 29.5.
We used the risk scores to stratify the risk of events into 3 groups (tertiles) and calculated the CIF.
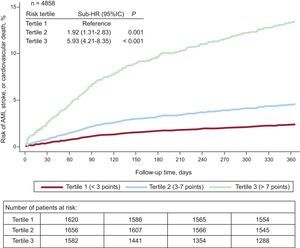
Of the 329 composite events during the first year, 39 occurred in the first tertile or low-risk group (< 3 points; CIF=2.5 [95%CI, 1.8-3.4]/100 person-years), 76 in the second tertile or intermediate-risk group (3-6 points; CIF=4.8 [95%CI, 3.8-6.0]/100 person-years), and 214 in the third tertile or high-risk group (> 6 points; CIF=15.5 [95%CI, 13.6-17.7]/100 person-years; P<.001) (Figure 3).
Cumulative incidence function of acute myocardial infarction, stroke, or cardiovascular death during the first year of follow-up stratified by risk tertiles. Adjusted for mortality as a competing event. 95%CI, 95% confidence interval; AMI, acute myocardial infarction; HR, hazard ratio.
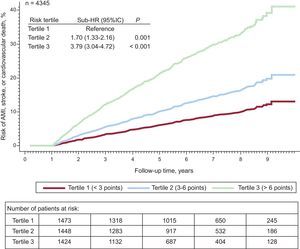
In the cohort that had composite events in successive years, the CIF in the first tertile (low-risk, < 3 points), the second tertile (intermediate risk, 3-6 points), and the third tertile (high risk, > 6 points) was 10.7 (95%CI, 8.4-13.0)/100 person-years (n=104 events), 19.0 (95%CI, 16.0-22.0)/100 person-years (n=167 events), and 40.3 (95%CI, 36.0 to 44.5)/100 person-years (n=345 events), respectively (P<.001) (Figure 4).
Cumulative incidence function of acute myocardial infarction, stroke, or cardiovascular death after the first year of follow-up stratified by risk tertiles. Adjusted for mortality as a competing event. 95%CI, 95% confidence interval; AMI, acute myocardial infarction; HR, hazard ratio.
The discriminative ability of the multivariable models was similar to that of the risk scores for the first year (0.74 [95%CI, 0.71-0.76] vs 0.73 [95%CI, 0.71-0.76]) and for successive years (0.69 [95%CI, 0.67-0.72] vs 0.68 [95%CI, 0.65-0.70]).
The discriminative ability of the risk score for AMI, stroke, or cardiovascular death during successive years remained virtually unchanged when the second follow-up period was restricted to 1, 3 or ≥ 5 years of follow-up (C statistic between 0.67 and 0.69).
The C statistic was 0.67 for the subgroups with LVEF < 35% and 35% to 45%, and 0.68 for subgroups with LVEF > 45% or unavailable LVEF, which are similar to the C statistic for the total group.
Both risk scores had good calibration (Hosmer-Lemeshow, P≥.44) ().
DISCUSSIONThis study shows that about 1 in 4 patients who are discharged from hospital after surviving an ACS will experience AMI, stroke, or cardiovascular death within the following 5 years. The risk of experiencing 1 of these events is particularly high in the first year, as shown by the finding that 34.8% (n=329) of the total number of events (n=945) occurred during the first 5±2 years of follow-up.
The results also show that the risk of AMI, stroke, or cardiovascular death decreases in successive years, but considerable residual risk remains such that 1 in 5 patients free of composite events during the first year will experience an event during the following 5 years.
Between 2006 and 2011, a Swedish study included more than 90 000 patients with AMI, of whom around 50% had undergone revascularization. One year after discharge, 18.3% of the patients experienced AMI, stroke, or cardiovascular death. At 3 years of follow-up, 20% of the patients who remained free of events during the first year experienced an event.9 However, between 2003 and 2004, the REACH registry20 included 21 890 patients with a history of ACS. Patients were followed-up until 2009. The cumulative incidence of AMI, stroke, or cardiovascular death was around 6% at 1 year and around 16% at 4 years. Similarly, between 2005 and 2010, Rapsomaniki et al21 studied more than 15 000 patients with ACS in the United Kingdom and found that the cumulative incidence of AMI, stroke, or cardiovascular death was 7.3%, 12.3%, and 17.7% at 1, 2, and 3 years, respectively. Thus, our results are in line with those recently obtained in different settings. All these results suggest that there is a high risk of recurrence of major cardiovascular complications after surviving the hospital phase of ACS.
Although there has been a recent decrease in mortality from ischemic heart disease,2,12–14 admissions for ACS will increase in Spain in the near future.12 The main cause of this increase will be the growing numbers of elderly people, which will account for up to 60% of all ACS patients by 2049.12 In addition to a greater mortality burden, the economic burden will also increase,22 because ACS care consumes large quantities of resources. At the beginning of the last decade, the direct costs alone of health care during the first year after an ACS were more than € 1 billion.22 Therefore, the management of chronic heart disease is a challenge for all the health services.
To meet this challenge, resources should be appropriately allocated to secondary prevention to reduce the high risk of future cardiovascular complications. Thus, it is essential to have data on the clinical course of the disease in its various stages and on the precursors of future adverse events, because the correct identification of the prognostic determinants would allow preventive therapies to be increased and allocated to high-risk patients, who are the most likely to benefit.23–28
In the present series, the predictors of AMI, stroke, or cardiovascular death comprised the interaction of classic risk factors (age, smoking, and diabetes mellitus), markers of atherosclerotic burden (previous ischemic heart disease, previous stroke or transient ischemic attack, peripheral artery disease, and renal impairment), and indicators of the extent of coronary artery disease (non—ST-segment elevation AMI and heart failure). Six of the 10 determinants of prognosis after the first year were also determinants during the first year, although the magnitude of the individual effect of these determinants varied according to the period studied. This study also shows that the proposed scoring systems can be used at the bedside or in the office to quantify the individual risk of AMI, stroke, or cardiovascular death.
All the predictors of events identified in our registry have already been previously described.9,11,20,29 The fact that smoking was associated with events during the first year but not during successive years may be because smoking cessation reduces or stops atherosclerotic plaque destabilization caused by smoking. However, this mechanism must remain speculative because no data were available on smoking after discharge. The reason for a history of atrial fibrillation being a predictor of events in the first year alone may be because the antithrombotic treatment strategy for these patients is not well defined and the effects are noticeable during the first months after discharge.
The scoring system for predicting events at 1 year of follow-up had acceptable discriminative power and good calibration. In contrast, the scoring system for predicting events in successive years had moderate discriminative power (0.68). Aside from these issues, we attempted to provide a simple method to identify prognostic determinants such that clinicians can become familiar with these determinants and incorporate them into clinical decision-making, thus helping them to stratify risk to guide prognostic strategies of proven efficiency23–28 and optimize follow-up strategies in cardiac rehabilitation programs.27
Although current financial constraints make it difficult to improve health resource allocation, such improvement is essential to address the vulnerability factors for various diseases. Individual stratification of long-term prognosis is needed to efficiently manage patients with chronic diseases. This study provides information on the magnitude of the impact of coronary disease and its predictors in a contemporary cohort of ACS patients. The scoring systems proposed in this study are not intended to replace clinical judgment, but to help the clinician to make well-reasoned balanced clinical judgments to optimize patient management and resource use.
LimitationsFirstly, this was a retrospective study with the limitations inherent to this type of design. For example, it cannot be confirmed that the causes of cardiovascular death were not underestimated. A notable strength of the study is that data were collected and processed by clinical cardiologists, thus providing qualitatively reliable data on which to base conclusions. A major limitation is the lack of an external validation analysis of the risk estimates of the scoring systems proposed in this study. These risk estimation systems should be validated, preferably prospectively, prior to their use in other populations of ACS patients.
Due to the sample size, some risk subgroups, especially those at the extremes of the risk scores, were not adequately represented and so risk should be estimated with caution.
Finally, because of the lack of data on treatment during follow-up, the differences observed between groups may have been at least partly due to differences in treatment.
CONCLUSIONSThe risk of the recurrence of cardiovascular events remained elevated in this contemporary cohort of ACS patients with a high rate of coronary revascularization. Based on a group of readily available predictors, risk can be easily quantified with acceptable predictive ability. Individual stratification of patients into groups at low or high risk of long-term events facilitates efficient resource use and the development of evidence-based chronic disease management programs.
CONFLICTS OF INTERESTNone declared.