Early detection of atrial fibrillation (AF) is a priority to reduce embolic events by initiating oral anticoagulation therapy. The aim of this study was to evaluate the diagnostic ability of a wrist device designed for automatic AF detection.
MethodsRITHMI is a prospective, comparative, observational study that included 167 patients referred to a cardiology outpatient clinic for a general consultation or for electrical cardioversion. The study evaluated the ability of a wrist monitor that uses a photoplethysmography (PPG) signal and an electrocardiographic lead to automatically detect AF compared with diagnosis established by 2 cardiologists using the 12-lead electrocardiogram.
ResultsThe AF detection algorithm based on the PPG signal had a sensitivity of 91% and a specificity of 96% (diagnostic accuracy: 93%). The automatic algorithm based on the electrocardiographic signal had a sensitivity of 94% and a specificity of 96% (diagnostic accuracy: 95%). The 2 algorithms concurred in the diagnosis in 96% of the cases. Overall, the monitor had a sensitivity and specificity of 95% (diagnostic accuracy: 95% and Kappa index: 0.98).
ConclusionsThis study shows that automatic AF detection through the use of a heart rhythm monitor incorporating sensors and algorithms that analyze the PPG signal and the electrocardiographic signal corresponding to lead I is feasible and has high diagnostic accuracy.
Keywords
Atrial fibrillation (AF) is the most common arrhythmic disorder in the general population. It affects between 3% and 4% of adults older than 20 years1 and is associated with an increased risk of death and heart failure in both men and women. It also increases the risk of stroke, cognitive impairment, and impaired quality of life. Early detection is essential as anticoagulant therapy can reduce mortality in patients at higher risk.2
The clinical presentation of AF is highly heterogeneous and can range from limiting symptoms to no symptoms at all (silent AF). Silent AF is common in patients with arrhythmia, particularly if they are elderly, and it increases the risk of stroke and heart failure.3 Its prevalence is unknown, although it has been shown that one-third of patients diagnosed with AF are asymptomatic.3 In addition, arrhythmia has been detected in 25% to 50% of patients admitted for AF-associated ischemic stroke.4 These findings indicate that silent AF is more common than believed and highlight the importance of early diagnosis, as initiation of anticoagulant therapy can prevent stroke.
AF detection has improved in recent years due to the use of electrocardiographic (ECG) monitoring systems in addition to 12-lead ECG. Several diagnostic systems have been validated and approved for clinical use, including heart rhythm recorders that require patient participation and prolonged ECG monitoring systems in the form of wearable skin patches or implantable devices.2 Finally, new technologies, such as smartphone cases with built-in ECG electrodes, have been found to be superior to routine clinical care for the detection of AF.5 All these studies concur that greater ECG monitoring intensity and duration improve AF detection.
An ideal AF detection tool should be noninvasive, accurate, passive (ie, does not require user intervention), not limited to isolated recordings, and capable of real-time detection. Wearable devices, in particular smartwatches and other wrist devices, are an attractive option in this regard and they are also cost-effective.6 Current generations of smartwatches are fitted with photoplethysmography (PPG) sensors capable of continuous heart rate monitoring. This technology has many applications and could be used as a noninvasive means of detecting AF through prolonged, heart rhythm monitoring.
The aim of this study was to evaluate the diagnostic ability of a wrist-wearable device designed to monitor heart rhythm and automatically detect AF via a dual-detection system based on PPG and ECG signals.
MethodsStudy design and patientsRITHMI is a prospective, nonrandomized study conducted at Hospital Universitario y Politécnico La Fe in Valencia, Spain between January and June 2019 to analyze the ability of the RITHMI wrist-wearable heart rhythm monitor to detect AF in patients older than 18 years referred to a cardiology outpatient clinic for cardioversion of AF or for a general consultation (patients with sinus rhythm or AF). We excluded patients with atrial flutter or an implanted pacemaker.
The study was carried out in accordance with the principles of the Declaration of Helsinki and was approved by the ethics committee of our hospital. All the patients provided signed informed consent agreeing to participate. A reference standard 12-lead ECG interpreted by 2 electrophysiologists was performed in all patients to determine whether they had sinus rhythm or AF.
After the 12-lead ECG, the RITHMI heart rhythm monitor was placed on the patients’ wrists and used to record their heart rhythm for 3minutes while they were seated. Patients seen for a general cardiology consultation continued to wear the wrist device, while those who underwent cardioversion did not.
RITHMI heart rhythm monitorThe wrist-wearable RITHMI heart rhythm monitor is a not yet commercially available prototype that is equipped with a dual-detection system involving 2 types of signals: heart rhythm signals detected by PPG in the distal region of the forearm/wrist and a single-lead ECG signal incorporated into the device that is equivalent to the I lead in 12-lead ECG. The PPG sensor (ADPD107) consists of 2 light-emitting diodes and a receiver on the underside of the device. The ECG sensor (AD8233) has 3 electrodes: 2 on the upper side of the device, which are placed on the index finger and thumb, and 1 on the underside of the device that is in contact with the skin. The position of the individual is designed to close the electrical circuit between the arms and provide an ECG recording equivalent to that provided by a standard I lead (figure 1). One of the electrodes on the upper side of the ECG sensor provides earthing to the circuit and helps eliminate electrical interference. The monitor has a low-consumption Bluetooth microcontroller and a data storage memory device.
The RITHMI monitor has 2 automated algorithms—a PPG and an ECG algorithm—programmed to detect AF by statistically analyzing signal irregularities. The PPG sensor is designed for continuous, passive heart rhythm monitoring, while the ECG sensor is used to confirm the presence of arrhythmia and generate an exportable graph for further analysis.
Statistical analysisContinuous variables are expressed as mean±SD and categorical variables as absolute values and percentages. The chi-square test was used to compare categorical variables and the t test to compare continuous quantitative variables.
The main aim of this study was to evaluate the ability of the RITHMI monitor to detect AF by comparing the diagnoses provided by the PPG and ECG algorithms with that provided by the reference standard 12-lead ECG interpreted by the 2 electrophysiologists. The evaluation was double-blinded: electrophysiologists were unaware of the results offered by the RITHMI heart rhythm monitor, while the engineer responsible for entering these results into the database was unaware of the reference standard diagnosis. At the end of the study, the diagnosis provided by each of the 2 algorithms was compared with the 12-lead ECG diagnosis.
The following variables were calculated for the PPG and the ECG algorithm to assess the ability of the RITHMI monitor to detect AF: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (sensitivity/(1-specificity), and negative likelihood ratio (1-sensitivity/specificity). We also calculated the percentage of correctly diagnosed cases, defined as the number of cases correctly diagnosed as AF plus the number of cases correctly diagnosed as sinus rhythm divided by the total number of cases and multiplied by 100.
The κ statistic was used to measure the level of agreement between the 2 automated AF detection systems (PPG and ECG) and between each of these systems and the reference standard 12-lead ECG.
ResultsThe ability of the RITHMI heart rhythm monitor to detect AF was evaluated in 167 patients, 109 of whom underwent cardioversion and 58 who were seen for a general cardiology consultation. The reference standard 12-lead ECG showed that 98 patients had AF and 69 had sinus rhythm.
The baseline characteristics of the patients are shown in table 1. Hypertension, diabetes, dyslipidemia, and a history of smoking were more common in patients with AF than with sinus rhythm at the time of analysis. The 2 groups were comparable in terms of mean age, sex, and presence of structural heart disease. Patients with AF had a mean±SD heart rate of 81 beats per minute (bpm) (range, 49-153 bpm).
Characteristics of the study population
| Sinus rhythm, No. (%) | AF, No. (%) | P value | |
|---|---|---|---|
| Age, y | 54±14 | 67±10 | <.001 |
| Sex, M/F | 64 (63)/38 (37) | 42 (61)/27 (39) | <.804 |
| Hypertension | 27 (39) | 55 (54) | <.058 |
| Diabetes | 7 (10) | 19 (19) | <.130 |
| Dyslipidemia | 13 (19) | 29 (28) | <.153 |
| Cardioversion/previous ablation | 15 (22) | 19 (19) | <.617 |
| Pacemaker or IAD | 5 (7) | 6 (6) | <.721 |
| Smoker or former smoker | 13 (19) | 27 (26) | <.248 |
| Atrial flutter | 12 (17) | 11 (11) | <.214 |
| Other heart disorders | 20 (29) | 15 (15) | <.002 |
| CHA2DS2-VASc | |||
| Anticoagulants | 37 (54) | 87 (85) | <.001 |
| Vitamin K antagonists | 14 (38) | 26 (30) | .371 |
| DOACs | 23 (62) | 61 (70) | .001 |
AF, atrial fibrillation; DOACs, direct oral anticoagulants; F, female; IAD, implantable automatic defibrillator; M, male.
Values are expressed as No. (%) or mean±SD.
The PPG algorithm was evaluated in 167 patients and the ECG algorithm in 133.
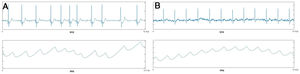
Ability of the PPG algorithm to detect AFThe PPG signal was uninterpretable in 5 of the 167 patients due to poor signal quality. In the remaining 162 patients, the algorithm diagnosed AF in 95 patients (figure 2A) and sinus rhythm in 67 (figure 2B).
The PPG algorithm showed a sensitivity of 91% and a specificity of 96% for the detection of AF compared with the reference standard 12-lead ECG. In addition, it had a PPV of 97%, an NPV of 88%, a diagnostic accuracy of 93%, a positive likelihood ratio of 20.22, a negative likelihood ratio of 0.05, a false-positive rate of 3%, and a false-negative rate of 9%. Subsequent analysis showed that the false positives were due to poor signal quality, with artifacts caused by sudden movement. The false negatives were attributed to variations in heart rate during arrhythmia that were below the threshold to be classified as an arrhythmic pulse. These variations were usually due to a high heart rate (mean, 121±32 vs. 68±14 bpm for patients with AF correctly identified by the device, P=.015).
Ability of the ECG algorithm to detect AFThe diagnostic ability of the RITHMI ECG algorithm was evaluated in 133 patients. It diagnosed AF in 87 patients and sinus rhythm in 46. Based on the reference standard diagnosis, it had a sensitivity of 94%, a specificity of 96%, a PPV of 98%, an NPV of 90%, a diagnostic accuracy of 95%, a positive likelihood ratio of 21.68, a negative likelihood ratio of 0.01, a false-positive rate of 2%, and a false-negative rate of 6%. The false positives were explained by signal artifacts due to poor contact between the electrodes and the skin resulting in abrupt changes in the electrical signal, while the false negatives were attributed to variations in heart beat during arrhythmia that were below the threshold to be classified as an arrhythmic pulse.
Combined diagnostic ability of the PPG and ECG algorithmsOverall, the PPG and ECG algorithms concurred in their diagnosis of AF or sinus rhythm in 96% of cases (κ=0.91). They produced discrepant results for either AF or sinus rhythm in 5 cases due to signal artifacts caused by movement of the patient during PPG recording or by poor contact between the sensor and the skin during ECG recording.
Overall (both algorithms provided the same diagnosis), the RITHMI monitor had a sensitivity and a specificity of 95% for the diagnosis of AF, a PPV of 99%, an NPV of 100%, a diagnostic accuracy of 95%, a positive likelihood ratio of 19.09, and a negative likelihood ratio of <0.1. The level of agreement between the diagnosis provided by the RITHMI monitor and the reference standard 12-lead ECG diagnosis was κ=0.98.
DiscussionThe RITHMI study evaluated the diagnostic ability of a new wrist-wearable AF detection monitor featuring 2 automated heart rhythm detection algorithms: one that analyzes PPG signals and another that analyzes single-lead ECG recordings equivalent to I lead recordings in 12-lead ECG. The results of the algorithms were compared with the reference standard 12-lead diagnosis established by 2 electrophysiologists. The RITHMI monitor diagnosed AF with an overall accuracy of 95% (sensitivity and specificity of 95%) in 167 patients (98 with AF) who were referred for a general cardiology consultation or cardioversion. This performance is superior to that reported for other AF detection methods, such as pulse palpation (72% specificity)7 and sphygmomanometry (specificity of 89%-91%).8,9
Compared with the reference standard diagnosis, the PPG algorithm had a diagnostic accuracy of 93% (91% sensitivity and 96% specificity) and a false-negative rate of 9%, attributed to a high heart rate (mean±SD, 121±32 bpm) during monitoring. These results are similar to those of other studies reporting a sensitivity of between 90% and 96% and a specificity of between 85% and 99% for smartphone PPG-based AF detection algorithms.10–12 The only study comparable to the RITHMI study, however, is the WATCH AF study, which analyzed the diagnostic ability of an algorithm based on signals emitted by PPG sensors available in several smartwatches on the market.13 In the WATCH AF study, the diagnosis provided by the algorithm was compared with that provided by a single-lead ECG sensor in the Kardia Band system (AliveCor Inc) that was interpreted by 2 cardiologists blinded to the results of the algorithm. The study included 508 patients (237 with AF). The algorithm had a sensitivity of 93.7%, a specificity of 98.2%, and a diagnostic accuracy of 96.1%. Both studies confirmed the presence of AF by ECG (single-lead ECG in the WATCH AF study and 12-lead ECG in the RITHMI study). They also showed a similar diagnostic ability for the PPG algorithm. The PPG signal could not be analyzed in 21.8% of the datasets in the WATCH AF study due to poor signal quality. This rate is clearly higher than that observed in our study (3%), highlighting the ability of the RITHMI monitor to provide PPG signals with sufficient quality for analysis. Obtaining an adequate PPG signal is the main technical limitation of wrist-wearable devices, hence the attractiveness of using a second system that detects AF via a different biological signal.
The RITHMI study also evaluated the usefulness of the ECG algorithm, which detects AF by analyzing single-lead ECG recordings. Based on the reference standard 12-lead ECG diagnosis, the ECG algorithm had an overall diagnostic accuracy of 95% (94% sensitivity and 96% specificity). These results can be compared with those of a study that evaluated the AliveCor Kardia Band automated ECG algorithm in a consecutive series of patients presenting for cardioversion.14 The study analyzed 169 simultaneous ECG and Kardia Band recordings obtained from analyzed 100 patients. Fifty-seven (33.7%) of the recordings analyzed by the automated Kardia Band algorithm were unclassifiable for various reasons, including, among others, artifacts, low amplitude signals, and abnormally high or low heart rates. For the remaining 112 recordings, the algorithm showed a sensitivity of 93% and a specificity of 84% for AF detection. These rates are lower than those observed for the ECG algorithm in the RITHMI heart rhythm monitor. The performance of the RITHMI ECG algorithm is comparable to that of algorithms used in implantable Holter monitors. The XPECT trial, for example, reported a sensitivity of 96.1% and a specificity of 85.4% for the Reveal LINQ algorithm (Medtronic) used by an implantable cardiac monitor.15
The dual-detection system of the RITHMI monitor showed an overall sensitivity and specificity of 95% for the detection of AF. This system is particularly attractive as the PPG and ECG sensors provide complementary results. Unlike other systems, the PPG sensor allows for continuous monitoring without the need for patient intervention. If it detects AF, the ECG sensor can be used to corroborate this result and provide a graph for further analysis.
AF detection and implications for the futureThe European Society of Cardiology guideline recommends opportunistic pulse-taking to detect silent AF in people older than 65 years (class I recommendation, level of evidence B) and systematic ECG screening in people older than 75 years or patients with a high risk of stroke (class IIa recommendation, level of evidence B).1
The technology described in this study could improve the diagnosis of AF in at-risk populations as it allows for continuous, passive monitoring via a wrist-wearable device. Before it can be widely used, however, it is first necessary to overcome the limitations associated with poor signal quality (noise artifacts, use of single-lead ECG recordings) and, above all, to confirm our findings in the general population, where there is a lower prevalence of AF and a higher likelihood of false positives. One recent study of over 400 000 people without a history of AF who had a smartphone and smartwatch with a dedicated AF application reported irregular pulse notifications consistent with AF in 0.52% of the overall population and in 3.2% of people older than 65 years.16 An ECG performed a mean of 13 days after the detection of irregularities confirmed the presence of AF in 34% of cases.
The possibility of using smartphone or smartwatch technology to detect AF could revolutionize the conventional model used to detect this disorder, blurring the distinction between patient and consumer and almost certainly leading to an increase in the demand for healthcare services and probably the number of people diagnosed with AF. At the same time, accessibility issues make it difficult to predict the role that this technology will play in the future and will also change the profile of people who will benefit from monitoring. Finally, studies are needed to investigate the usefulness of anticoagulant treatment in patients with AF detected by smart technology.
LimitationsThe main limitation of the study is linked to the conditions in which the PPG and ECG signals were obtained, as it is easier to obtain an adequate signal (particularly in the case of the PPG sensor) in patients at rest and in environments free of external stimuli. The limitations associated with measuring electrical rhythms captured by PPG sensors are described in the literature, and include the influence of tremor or motion on the signal-to-noise ratio.10–13 All the patients in our series were white, so we were unable to evaluate the potential influence of skin pigmentation on signal quality and algorithm performance. The PPG algorithm was associated with a false-negative rate of 9% and most of the false negatives were due to a high heart rate. Other studies have not described heart rate or have excluded patients with higher rates13,14 because of their effect on automated AF detection algorithms.
Overall, we were unable to analyze the performance of the PPG algorithm in 3% of patients due to poor signal quality signal. This rate, however, might have been higher in a less controlled environment. Patients with atrial flutter or a pacemaker were excluded from our study, as they may have a regular pulse (the RITHMI algorithms work by analyzing irregularities).
The ECG algorithm was analyzed in just 133 patients as it was incorporated into the RITHMI monitor at a later data than the PPG algorithm. It is unlikely, however, that earlier incorporation would have significantly modified our results.
Another limitation of our study is our small sample size. It would be desirable to confirm our results in a larger population exposed to different conditions (ie, not only in patients at rest or in a controlled environment). Further studies are needed before the RITHMI monitor can be validated for use in an AF detection campaign. Nonetheless, our findings do confirm proof of concept and serve as a starting point for designing a study to assess the usefulness of this wrist-wearable device in the general population.
ConclusionsThe results of the RITHMI study show that continuous, automated detection of AF by means of a heart rhythm monitor incorporating sensors and algorithms that analyze PPG signals and single-lead ECG recordings equivalent to those provided by the I lead in 12-lead ECG is feasible and has high diagnostic accuracy.
Conflicts of InterestC. Planells Palop is a signal processing engineer and J. Osca Asensi a medical consulant at Arrythmia Algorithm S.L.
- -
AF detection has improved in recent years due to the use of ECG monitoring systems aside from 12-lead ECG.
- -
The use of new technologies, in particular smartphones, smartwatches, and smart wearable devices, could provide a noninvasive, continuous monitoring system capable of detecting AF in real time.
- -
The RITHMI study has shown the usefulness of a new heart rhythm monitor incorporating 2 automated algorithms that can detect AF by analyzing PPG and ECG signals.
- -
Both the PPG and ECG algorithms showed good diagnostic accuracy.
- -
Overall, the RITHMI heart rhythm monitor could be used for passive continuous heart monitoring via analysis of PPG signals and confirmation of results by ECG analysis.