Heart failure (HF) is a major regional and global problem afflicting over 5 million Americans. According to the American Heart Association, about 550 000 new cases are diagnosed each year. Among these patients, approximately 250 000 have advanced HF and are still symptomatic despite maximal medical therapy. HF is the leading cause of hospitalizations, which poses a huge national financial burden with over USD 33 billion spent on this disease alone in 2007.1 The number of patients with chronic HF continues to rise rapidly due to advances in health care resulting in people living longer. In addition, the treatments for acute episodes of decompensated HF, myocardial infarction, cardiac arrest and postcardiotomy cardiogenic shock have significantly improved. For many of these patients cardiac transplantation would be the preferred treatment option. Unfortunately, as the list of patients requiring cardiac transplantation continues to grow, the donor pool of usable hearts has remained stagnant. Therefore, cardiac transplantation is a very limited option which is reserved for only a fraction of the patients with advanced HF. Mechanical circulatory support, particularly implantable durable left ventricular assist devices (LVADs), is starting to fill this treatment void for patients with advanced HF that no longer respond to optimal medical therapy.
Ventricular assist devicesThese blood propelling devices have evolved rapidly over the past decade (Figure 1). As they continue to change in form and size, they can currently be divided into 2 groups based on flow characteristics: pulsatile flow and continuous flow (CF) pumps.
Figure 1. Evolution of mechanical circulatory support devices from large external pulsatile flow devices to small internal continuous flow devices. Ao, aorta; LVAD, left ventricular assist device; PA, pulmonary artery; RA, right atrium; RVAD, right ventricular assist device. Reproduced with permission from Thoratec Corp, Pleasanton, CA, United States.
The first generation ventricular assist devices are pulsatile positive displacement pumps or pulsatile flow pumps. The HeartMate XVE, Thoractec PVAD (Thoratec Corporation, Pleasanton, CA, United States), Novacor (WorldHeart, Oakland, CA, United States) and the Berlin Excor (Berlin Heart, Berlin, Germany) are current examples of pulsatile flow devices. The HeartMate XVE was the first device approved for destination therapy (DT) following the completion of the REMATCH (Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure) trial.2 More recent LVADs for clinical use are based on CF technology. They can be divided into 2 groups, either axial flow or centrifugal flow, depending on the flow pattern. These pumps have minimal touching parts and in some the impeller is completely magnetically levitated so that there are no touching parts within the system. Examples of CF LVADs would include the HeartMate II (Thoratec Corp, Pleasanton, CA, United States), Jarvik 2000 (Jarvik Heart, New York, NY, United States), Berlin Heart Incor (Berlin Heart, Berlin, Germany) and HeartAssist5 (MicroMed, Houston, TX, United States). More recent CF pumps that are currently in clinical trials in the United States include the HVAD (HeartWare International, Farmingham, MA, United States) and DuraHeart (Terumo Heart, Ann Arbor, MI, United States). The CentriMag (Thoratec Corporation, Pleasanton, CA, United States) blood pump is a short-term CF extracorporeal circulatory support device providing hemodynamic stabilization in patients in need of cardiopulmonary support. It can also be used as a right ventricular assist device or in an extracorporeal membrane oxygenation configuration. By design, CF pumps provide continuous forward flow as well as unloading. Therefore, patients supported with a CF pump will have a diminished pulse pressure and sometimes no pulse at all.
Use of left ventricular assist device in advanced heart failureInitially, left heart support with mechanical devices was provided to patients with end-stage HF as a “bridge to transplantation” (BTT). Their long term use for the purposes of enhancing survival and improving quality of life is now an acceptable alternative in patients who are not candidates for cardiac transplantation. Hence, these devices now have diverse roles. They are still utilized as BTT but are increasingly being used as DT or permanent support. A new exciting role of “bridge to recovery” is currently being investigated and in some cases, these devices are used as a “bridge to decision”.
Bridge to transplantationCardiac transplantation has been shown to increase the quality and duration of life in patients with end-stage HF. Unfortunately, there are only a finite number of good donor hearts. Conversely, mechanical support systems, in theory, are limitless. There are different constraints on the use of ventricular assist devices namely economic, patients’ proximity to implant centers, adequate home support and acceptance by the community at large. Although there has never been a randomized trial comparing the use of ventricular assist devices as an alternative to transplantation, support from these devices reduces mortality and improves the patient's overall condition before and after transplantation with 70% of patients surviving to transplantation.3 They improve renal function, nutritional status and pulmonary vascular resistance, which may require several months. These early trials were conducted with the first generation pumps, which were associated with higher mortality and morbidity rates. Use of the second generation pumps resulted in even better outcomes. Two large prospective multicenter trials concluded that almost 80% of patients achieved the principal outcomes of transplantation, cardiac recovery or successful ongoing support with continued transplant eligibility. The patients had a lower risk of sudden death while awaiting transplantation and could return to a fairly normal lifestyle.4, 5 The third generation devices have continued to improve this trend with an actual survival at 6, 12, and 24 months of 90%, 84%, and 79%, respectively.6
Destination TherapyThe concept of DT has only evolved over the past 10 years after encouraging results were obtained from the BTT trials. Candidates for this option are by definition ineligible for cardiac transplantation due to a combination of increased age and other comorbidities. Hence, they are usually in poor health with a limited life expectancy. Even in these patients, those with LVADs have had superior outcomes compared to those treated with maximal medical therapy alone. In a post-REMATCH analysis, the 1-year survival rate for these patients was 61% compared to 25% for the REMATCH medical therapy group.7
The early DT experience was limited by device durability and the size of the pulsatile device, which limited the widespread acceptance of this therapy. Newer systems have since been developed (CF LVADs) which provide safe and effective circulatory support with significantly improved durability and reduced size. They have superior results compared to the first generation pumps with 1- and 2-year survival rates of 68% and 58%, respectively.8 A recent report from INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) revealed an even wider gap in 1-year survival rates between patients supported with pulsatile devices (61%) and patients with CF devices (74%).9
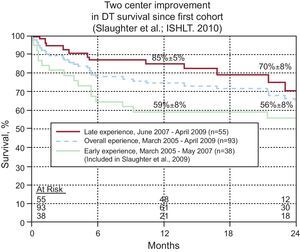
These results will continue to improve as patient selection becomes more refined (Figure 2). The sickest patients obviously do the worst so the dilemma is in identifying the boundary between the patients that are too ill and those that are too well. The INTERMACS data has consistently shown that patients in the sickest category have the highest mortality.9 Implanting a device into these patients should be avoided or delayed until they are stabilized and their condition improved. Predictive models can be employed to predict outcomes of HF patients. The presence of comorbidities requires careful consideration in selecting appropriate candidates for DT. Preexisting conditions not related to HF that limit survival to<2 years are generally viewed unfavorably. Other important factors include the use of illicit drugs, poor home environment without social support and psychiatric disorders. Conditions that are treatable such as active ongoing infection, coagulopathy, and poor nutritional state should be managed preoperatively. Other conditions such as valvular pathology can be treated intraoperatively. Postoperative right HF is probably the most difficult complication to predict but careful patient selection can help lower its incidence.10
Figure 2. Two-center improvement in destination therapy survival since the first cohort. As the surgical experience and patient selection have become more refined, so too have the outcomes in terms of patient survival. DT, destination therapy; ISHLT, The International Society for Heart and Lung Transplantation.
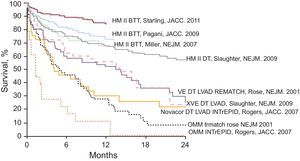
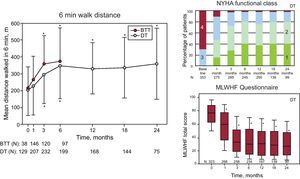
Due to improvements in technology, as well as patient selection and management, there has been a steady and significant improvement in survival for patients supported with a LVAD for both BTT and DT (Figure 3). Equally important to survival is the concomitant improvement in functional class and quality of life that these patients experience due to the improved hemodynamics (Figure 4).11 As a result of these improvements, outcomes for patients supported with a LVAD are approaching the same long-term results that have been achieved with the gold standard of heart transplantation.11
Figure 3. Improved survival in left ventricular assist device trials. Due to improvements in technology as well as patient selection and management, there has been a steady and significant improvement in survival for patients supported with left ventricular assist devices as both a bridge to transplantation and destination therapy. BTT, bridge to transplantation; DT, destination therapy; HM II, HeartMate II; INTrEPID, Investigation of Nontransplant-Eligible Patients Who Are Inotrope Dependent; LVAD, left ventricular assist device; OMM, optimal medical management; REMATCH, Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure; VE, Vented Electric; XVE, Extended Line Vented Electric. Reproduced with permission from Thoratec Corp, Pleasanton, CA, United States.
Figure 4. Patient functionality and quality of life on ventricular assist device therapy continue to improve with time. BTT, bridge to transplantation; DT, destination therapy; MLWHF, Minnesota living with heart failure; NYHA, New York Heart Association; QoL, quality of life; VAD, ventricular assist device. Reproduced with permission from Rogers et al. 11
Bridge to decisionOccasionally patients present in a state of cardiogenic shock and are moribund. In this acute setting it can be difficult to ascertain if the end-organ failure, including the neurological status, is reversible. These patients usually do very poorly and would not be candidates for a long-term device or emergent cardiac transplantation. If the contraindications are considered acute and potentially reversible or if there is potential for a full cardiac recovery, then the use of a short-term ventricular assist device can be life-saving. The Centrimag is a very effective, low cost device that can be used in this setting. It provides good hemodynamic support, improves end-organ function and affords the opportunity to further evaluate the patient's clinical condition.12 It can be inserted with relative ease and minimal dissection, which is important as these patients usually have a coagulopathy and are unstable. The support it delivers is immediate and if the clinical condition improves, the patient can then be considered for a longer term device, explantation or cardiac transplantation.
Bridge to recoveryThis is an exciting new use for LVADs. Previously a small number of patients supported with LVADs showed an improvement in cardiac function but there is currently more evidence that prolonged unloading of the left ventricle results in reverse remodeling and functional improvement, which in some cases allows LVAD explantation.13 The strategy for achieving this involves both mechanical and pharmacological therapy. Complete unloading of the left ventricle coupled with aggressive pharmacological support maximizes the incidence of recovery in patients with dilated cardiomyopathy and improves the durability of the recovery following explantation. Patients need to continue standard HF medical therapy while on device support and then undergo some form of a weaning protocol, which requires testing at low pump speeds. Patients that meet specific criteria can be offered the opportunity to have the LVAD removed for myocardial recovery. Strategies similar to this have resulted in excellent success with recovery resulting in LVAD explantation in over 70% of patients with nonischemic cardiomyopathy.13 These patients remain well, with better quality of life compared to transplant recipients 9 years later, suggesting that the recovery is also durable.14
SummaryLVADs to support patients that have failed medical therapy is now a successful treatment option for many patients. With the limited donor pool for heart transplantation, many of these patients will require prolonged support while waiting or will have the device as lifelong permanent support. Current CF LVADs are very durable and reliable and have helped to expand the field. Additional improvements in patient management and the blood contact interface with the device will further reduce the complications seen with long-term support. Improving technology and patient outcomes has allowed the use of current LVADs to approach the ultimate goal of providing a mechanical solution for advanced HF, and perhaps even before the development of worsening symptoms and increased hospitalizations.
Conflicts of interestDr. Slaughter: research/grant support Thoratec Corporation.
Corresponding author: 201 Abraham Flexner Way, Suite 1200, Louisville, KY 40202, United States. mark.slaughter@louisville.edu