Key sex differences have been explored in multiple cardiac conditions. However, sex impact in hypertrophic cardiomyopathy outcome is unclear. We aimed to characterize sex impact in overall and cardiovascular (CV) mortality in a nationwide hypertrophic cardiomyopathy registry.
MethodsWe analyzed 1042 adult patients, 429 (41%) women, from a national registry of hypertrophic cardiomyopathy, with mean age at diagnosis 53±16 years and a mean follow-up of 65±75 months. At baseline, women were older (56±16 vs 51±15 years; P <.001), more symptomatic (56.4%, vs 51.7%; P <.001) and had more heart failure (42.0% vs 24.2%. P <.001), diastolic dysfunction (75.2% vs 64.1% P=.001), moderate/severe mitral regurgitation (33.4% vs 21.7%; P=.003), and higher B-type natriuretic peptide levels (920 [366-2412] mg/dL vs 487 [170-1087] mg/dL; P <.001). Women underwent fewer stress tests and cardiac magnetic resonance.
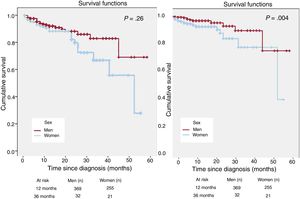
ResultsKaplan-Meier survival curves showed higher overall (8.4% vs 5.0%; P=.026) and CV mortality (5.5% vs 2.2%; P=.004) in women. Cox proportional hazard regression showed that female sex was an independent predictor of overall (HR, 2.05; 95%CI, 1.11–3.78; P=.021) and CV mortality (HR, 3.16; 95%CI, 1.25–7.99; P=.015). Women had more heart failure-related death (2.6% vs 0.8%, P=.024). Despite similar sudden cardiac death (SCD) risk, women received fewer implantable cardioverter-defibrillators (10.9% vs 15.6%; P=.032) and, in patients without cardioverter-defibrillators, SCD occurred more commonly in women (1.8% vs 0.4%; P=.031).
ConclusionsIn this nationwide registry, female sex was an independent predictor of overall and CV-related death, with more heart failure-related death. Despite similar SCD risk, women were undertreated with implantable cardioverter-defibrillators. These data highlight the need for an improved clinical approach in women with HCM.
Keywords
Hypertrophic cardiomyopathy (HCM) is defined by the presence of increased left ventricle (LV) wall thickness that is not explained by abnormal loading conditions.1 It has an annual incidence of 0.3 to 0.5 per 100 000 persons, and is most frequently transmitted as an autosomal dominant trait, although a small male sex preponderance has been described.2,3 Current guidelines suggest that this may reflect bias in screening strategies as well as genetic and hormonal modifiers.1
Sex differences have clear implications in many cardiac conditions.4–6 However, in HCM, these differences have not been clearly identified. Previous studies showed that women may not only be underrepresented in HCM cohorts, as they also seem to present in a later stage of the disease, with more left ventricular outflow tract (LVOT) obstruction and more symptoms.4 Recently, a study with an American-based population was the first to report that women with HCM may have a worse prognosis.7
We aimed to characterize sex differences in overall and cardiovascular (CV) mortality in a large European Union country-based nationwide HCM registry.8
MethodsThe Portuguese Registry of Hypertrophic CardiomyopathyThe Portuguese Registry of Hypertrophic Cardiomyopathy is an observational, retrospective, nationwide, multicenter registry. Inclusion criteria were age> 18 years at the time of enrolment and HCM defined according to the European Society of Cardiology, ie, unexplained LV hypertrophy (LVH) with maximum LV wall thickness ≥ 15mm by imaging techniques.1 Exclusion criteria were grade ≥ 2 hypertension, moderate or severe aortic stenosis, previously diagnosed cardiac or systemic disease, and metabolic or multiorgan syndrome associated with LVH. Centers were asked to include all patients with a diagnosis of HCM followed up at the center until April 2015, currently or in the past (no retrospective time limit), including those already deceased at the time of enrolment. Follow-up time was defined as the time from the initial assessment at the center to the last assessment or death. The Portuguese Registry of Hypertrophic Cardiomyopathy collected sociodemographic and clinical data regarding previous medical history, clinical presentation, diagnostic tests, phenocopy exclusion, treatment, follow-up, and events during the follow-up. LVOT obstruction was defined as the presence of a LVOT gradient ≥ 30mmHg. Written informed consent was obtained from living patients or from a proxy of deceased patients. This investigation conforms to the principles outlined in the Declaration of Helsinki and was approved by the National Center for Data Protection and local ethics committees. Further details regarding the Portuguese Registry of Hypertrophic Cardiomyopathy have already been published.8,9
Outcomes in hypertrophic cardiomyopathyTwo primary endpoints were defined: a) overall mortality and b) CV mortality during follow-up. CV mortality was defined as sudden cardiac death (SCD) or death related to stroke, myocardial infarction, or heart failure (HF). According to the 2016 European Society of Cardiology HF guidelines, HF was defined as the clinical syndrome caused by a structural and/or functional cardiac abnormality resulting in reduced cardiac output and/ or elevated intracardiac pressures at rest or during stress.10 SCD or SCD equivalent was a secondary endpoint defined as any SCD, successful resuscitation from SCD, or appropriate defibrillation therapies by an implantable cardioverter-defibrillator (ICD). Considering that, before 2014, HCM patients were managed according to the previous guidelines on ICD implantation for primary prevention of SCD, we applied the classic risk factors of SCD11 to estimate the SCD risk in patients with HCM. Additionally, we also evaluated the HCM Risk-SCD score and classified patients according to their SCD risk at 5 years (< 4%, ≥ 4 and <6%, or ≥ 6%).1
Statistical analysisCategorical variables are presented as frequencies and continuous variables as mean (μ)±standard deviation (SD) or median (x)±interquartile range [IQR]. Chi-square or Fisher tests were used for comparisons of categorical variables and Student t tests, ANOVA or the nonparametric equivalents were used for comparison of continuous variables. Cox proportional hazard regression analyses evaluated the relationship of sex with time of death from any cause or from CV cause. For survival analysis, a multivariate model with survival curves was constructed according to the Kaplan-Meier method, and comparisons were performed using the log-rank test. All P-values were 2-sided and were considered significant when <.05.
ResultsThis study included 1042 adult patients with HCM from 29 participating centers; 429 (41.2%) were women. The mean age at diagnosis was 53±16 years. Patients were followed up between 1975 and 2015 with a mean follow-up of 65±75 months. The number of cases per center and the demographic distribution by center has been published in a previous trial.8
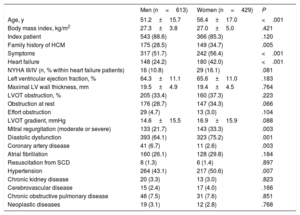
All relevant baseline characteristics at diagnosis, stratified by sex, are described in table 1. At the time of diagnosis women were older (56±16 vs 51±15 years, P<.001) and more symptomatic (56.4%, vs 51.7%; P <.001), complaining more frequently than men of dyspnea (47.7% vs 28.6%; P <.001) and palpitations (26.0% vs 17.6%; P=.002), but not syncope (10.3% vs 10.8%; P=.823). There were no differences relating to the prevalence of atrial fibrillation (11.2% vs 12.1%; P=.419). HF diagnosis was more common in women (42.0% vs 24.2%; P <.001), as was mitral regurgitation (moderate or severe in 33.4% vs 21.7%; P=.003) and diastolic dysfunction (75.2% vs 64.1% P=.001). Women had higher B-type natriuretic peptide levels at diagnosis (median 920 [IQR 366-2412] mg/dL vs median 487 [IQR 170-1087] mg/dL; P <.001). Hypertension was also more prevalent in women (50.6% vs 43.1%; P=.007), unlike coronary artery disease, which was more common in men (2.6% vs 6.7%; P=.003).
Baseline characteristics of patients with hypertrophic cardiomyopathy
| Men (n=613) | Women (n=429) | P | |
|---|---|---|---|
| Age, y | 51.2±15.7 | 56.4±17.0 | <.001 |
| Body mass index, kg/m2 | 27.3±3.8 | 27.0±5.0 | .421 |
| Index patient | 543 (88.6) | 366 (85.3) | .120 |
| Family history of HCM | 175 (28.5) | 149 (34.7) | .005 |
| Symptoms | 317 (51.7) | 242 (56.4) | <.001 |
| Heart failure | 148 (24.2) | 180 (42.0) | <.001 |
| NYHA III/IV (n, % within heart failure patients) | 16 (10.8) | 29 (16.1) | .081 |
| Left ventricular ejection fraction, % | 64.3±11.1 | 65.6±11.0 | .183 |
| Maximal LV wall thickness, mm | 19.5±4.9 | 19.4±4.5 | .764 |
| LVOT obstruction, % | 205 (33.4) | 160 (37.3) | .223 |
| Obstruction at rest | 176 (28.7) | 147 (34.3) | .066 |
| Effort obstruction | 29 (4.7) | 13 (3.0) | .104 |
| LVOT gradient, mmHg | 14.6±15.5 | 16.9±15.9 | .088 |
| Mitral regurgitation (moderate or severe) | 133 (21.7) | 143 (33.3) | .003 |
| Diastolic dysfunction | 393 (64.1) | 323 (75.2) | .001 |
| Coronary artery disease | 41 (6.7) | 11 (2.6) | .003 |
| Atrial fibrillation | 160 (26.1) | 128 (29.8) | .184 |
| Resuscitation from SCD | 8 (1.3) | 6 (1.4) | .897 |
| Hypertension | 264 (43.1) | 217 (50.6) | .007 |
| Chronic kidney disease | 20 (3.3) | 13 (3.0) | .823 |
| Cerebrovascular disease | 15 (2.4) | 17 (4.0) | .166 |
| Chronic obstructive pulmonary disease | 46 (7.5) | 31 (7.6) | .851 |
| Neoplastic diseases | 19 (3.1) | 12 (2.8) | .768 |
HCM, hypertrophic cardiomyopathy; LV, left ventricular; LVOT, left ventricular outflow tract; NYHA, New York Heart Association; SCD, sudden cardiac death.
Data are expressed as No. (%) or mean±standard deviation.
An electrocardiogram (ECG) was performed in all patients, and transthoracic echocardiography was performed in 99.7% (n=609) of men and 99.3% (n=426) of women (P=.654). However, women underwent fewer stress ECG (36.5% vs 46.6% P=.001), stress echocardiography (13.7% vs 19.4% P=.018) and cardiac magnetic resonance (41.7% vs 49.1%; P=.020). ECG, stress echocardiography and cardiac magnetic resonance data are presented in .
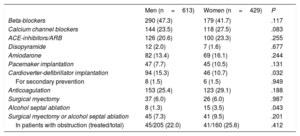
There was a trend to a higher frequency of LVOT obstruction at rest in women (36.3% vs 30.7%; P=.066). The frequency of LVOT obstruction at effort was similar in both sexes, but it could be underdiagnosed in women as they less frequently underwent stress echocardiography. Women underwent more alcohol septal ablation than men (3.5% vs 1.3% P=.018), but the frequency of myectomy was similar between sexes (6.0% vs 6.0%; P=.987). All therapeutic measures are summarized in table 2. All ICDs were implanted according to the current recommendations at the time of implantation.
Therapeutic interventions in patients with hypertrophic cardiomyopathy
| Men (n=613) | Women (n=429) | P | |
|---|---|---|---|
| Beta-blockers | 290 (47.3) | 179 (41.7) | .117 |
| Calcium channel blockers | 144 (23.5) | 118 (27.5) | .083 |
| ACE-inhibitors/ARB | 126 (20.6) | 100 (23.3) | .255 |
| Disopyramide | 12 (2.0) | 7 (1.6) | .677 |
| Amiodarone | 82 (13.4) | 69 (16.1) | .244 |
| Pacemaker implantation | 47 (7.7) | 45 (10.5) | .131 |
| Cardioverter-defibrillator implantation | 94 (15.3) | 46 (10.7) | .032 |
| For secondary prevention | 8 (1.5) | 6 (1.5) | .949 |
| Anticoagulation | 153 (25.4) | 123 (29.1) | .188 |
| Surgical myectomy | 37 (6.0) | 26 (6.0) | .987 |
| Alcohol septal ablation | 8 (1.3) | 15 (3.5) | .043 |
| Surgical myectomy or alcohol septal ablation | 45 (7.3) | 41 (9.5) | .201 |
| In patients with obstruction (treated/total) | 45/205 (22.0) | 41/160 (25.6) | .412 |
ACE, angiotensin converter enzyme; ARB, angiotensin receptor blocker.
Data are expressed as No. (%)
Genetic testing was performed in 51% of the population included (n=528). Of these, 59% were men and 41% women, with no statistically significant differences in the number of genetic tests performed between sexes (309/613 men, 50% vs 219/429 women, 51%; P=.96). Regarding testing results, 40% (n=210) of the tests identified a pathogenic/likely pathogenic mutation.9 The number of positive test results was also not different between sexes. All genetic data from our registry was analyzed in a recently published trial by Lopes et al.9 Active exclusion of phenocopies was more frequently performed in high-volume centers, but similarly between sexes, as previously published.8,9
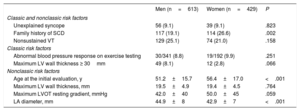
Women underwent less ICD implantation (10.9% vs 15.6%; P=.032, table 2) despite similar SCD risk according to classic and nonclassic risk factors for SCD (table 3). Regarding classic risk factors for SCD, men and women had the same prevalence of unexplained syncope (10.8% vs 10.3%; P=.823), nonsustained ventricular tachycardia (VT) (23.0% vs 19.5%; P=.193), LV maximum wall thickness ≥ 30mm (8.1% vs 2.8%; P=.066), and abnormal blood pressure response to exercise (8.8% vs 9.9%; P=.668), while women had more family history of SCD (28.6% vs 20.1%, P=.002). Overall, the mean number of classic risk factors for SCD was similar in men and women (0.7% vs 0.7%; P=.575), as well as the prevalence of patients with at least 1 classic risk factor (43.9% vs 46.8%; P=.352). Considering patients with 1 or more classic risk factors for SCD, and therefore potentially eligible for ICD, we observed that the overall ICD implantation rate was not high in either sex. However, we identified a statistically significant trend toward a lower implantation rate in women (23.4%, 46/197 vs 31.8%, 84/264; P=.045).
Risk factors for sudden cardiac death in patients with hypertrophic cardiomyopathy
| Men (n=613) | Women (n=429) | P | |
|---|---|---|---|
| Classic and nonclassic risk factors | |||
| Unexplained syncope | 56 (9.1) | 39 (9.1) | .823 |
| Family history of SCD | 117 (19.1) | 114 (26.6) | .002 |
| Nonsustained VT | 129 (25.1) | 74 (21.0) | .158 |
| Classic risk factors | |||
| Abnormal blood pressure response on exercise testing | 30/341 (8.8) | 19/192 (9.9) | .251 |
| Maximum LV wall thickness ≥ 30mm | 49 (8.1) | 12 (2.8) | .066 |
| Nonclassic risk factors | |||
| Age at the initial evaluation, y | 51.2±15.7 | 56.4±17.0 | <.001 |
| Maximum LV wall thickness, mm | 19.5±4.9 | 19.4±4.5 | .764 |
| Maximum LVOT resting gradient, mmHg | 42.0±40 | 50.0±45 | .059 |
| LA diameter, mm | 44.9±8 | 42.9±7 | <.001 |
LV, left ventricular; LVOT, left ventricular outflow tract; SCD, sudden cardiac death; VT, ventricular tachycardia.
Data are expressed as No. (%) or mean±standard deviation.
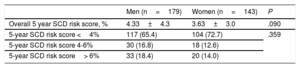
Regarding risk factors included in the currently recommended score for risk stratification of SCD–the HCM Risk-SCD score–men and women had a similar maximum gradient on LVOT at rest (42.0±40mmHg vs 50.0±45mmHg; P=.059), while men had larger left atrium (LA) diameter (42.9±7 vs 44.9±8mm; P <.001) and maximum LV wall thickness (17.7±4 vs 18.4±5mm; P=.041). As stated previously, women were older (56±16 vs 51±15 years; P<.001). However, SCD risk at 5 years was similar in men and women according to the HCM Risk-SCD score, with no differences on the mean score or on the risk stratification categories between sexes (table 4). In the group of patients potentially eligible for ICD, ie, those with an HCM Risk-SCD score ≥ 4%, women received fewer ICD than men and this difference also represented a statistically significant trend (12/38 women, 31.6% vs 29/63 men, 46.1%; P=.046).
Hypertrophic cardiomyopathy Risk-SCD score, stratified by sex
| Men (n=179) | Women (n=143) | P | |
|---|---|---|---|
| Overall 5 year SCD risk score, % | 4.33±4.3 | 3.63±3.0 | .090 |
| 5-year SCD risk score <4% | 117 (65.4) | 104 (72.7) | .359 |
| 5-year SCD risk score 4-6% | 30 (16.8) | 18 (12.6) | |
| 5-year SCD risk score> 6% | 33 (18.4) | 20 (14.0) |
SCD, sudden cardiac death.
Data are expressed as No. (%) or mean (±) standard deviation.
Mortality occurred in 6.3% (n=65), and CV mortality occurred in 3.7% (n=39). Kaplan-Meier analysis (figure 1) showed higher overall (8.4% vs 5.0%; P=.026) and CV (5.5% vs 2.2%; P=.004) mortality in women than in men. Higher overall mortality was also identified in patients with age at diagnosis> 60 years (6.1% vs 3.53%; P=.033), a positive family history of SCD (8.6% vs 5.1%; P=.016), and systolic dysfunction (9.8% vs 4.7%; P=.004). After multivariate modelling, including adjustment for mortality associated variables and all variables with statistically significant differences between sexes (presented in table 1), female sex remained independently associated with overall mortality (HR, 2.05; 95%CI, 1.11-3.75; P=.021) and CV mortality (HR, 3.16; 95%CI, 1.25–7.99; P=.015).
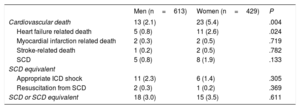
The higher overall and CV mortality in women was mainly due to a higher frequency of HF-related death (2.6% vs 0.8%; P=.024, table 5). SCD occurrence in women was numerically higher but was not statistically significant (1.9% vs 0.8% vs P=.133). There was also no statistically significant difference in SCD equivalents (3.0% vs 3.5%; P=.611, table 5).
Cardiovascular causes of death and sudden cardiac death equivalents in hypertrophic cardiomyopathy patients
| Men (n=613) | Women (n=429) | P | |
|---|---|---|---|
| Cardiovascular death | 13 (2.1) | 23 (5.4) | .004 |
| Heart failure related death | 5 (0.8) | 11 (2.6) | .024 |
| Myocardial infarction related death | 2 (0.3) | 2 (0.5) | .719 |
| Stroke-related death | 1 (0.2) | 2 (0.5) | .782 |
| SCD | 5 (0.8) | 8 (1.9) | .133 |
| SCD equivalent | |||
| Appropriate ICD shock | 11 (2.3) | 6 (1.4) | .305 |
| Resuscitation from SCD | 2 (0.3) | 1 (0.2) | .369 |
| SCD or SCD equivalent | 18 (3.0) | 15 (3.5) | .611 |
ICD, implantable cardiac defibrillator; SCD, sudden cardiac death.
Data are expressed as No. (%).
Due to the differences in ICD implantation rates between sexes, table 6 presents data regarding CV causes of death among patients with and without ICD. In patients with ICD (94 men and 46 women), no statistically significant differences were obtained. presents the baseline characteristics of patients with no ICD: women were more frequently diagnosed with HF (42.8% vs 26.9%; P=.002) and moderate to severe mitral regurgitation (19.2% vs 11.5%; P=.044). However, no other differences were identified by age (54±19 vs 49±18; P=.066) or by any comorbidities that could explain a lower ICD implantation rate.
Cardiovascular causes of death and sudden cardiac death equivalents in hypertrophic cardiomyopathy patients with and without implantable cardiac defibrillator
| With ICD | Men (n=94) | Women (n=46) | P |
|---|---|---|---|
| Cardiovascular death | 4 (4.4) | 3 (6.6) | .523 |
| Heart failure related death | 1 (1.1) | 2 (4.4) | .208 |
| Myocardial infarction related death | 0 | 0 | - |
| Stroke related death | 0 | 0 | - |
| SCD | 3 (3.2) | 1 (2.2) | .734 |
| SCD equivalent | |||
| Appropriate ICD shocks | 11 (11.7) | 6 (13.0) | .719 |
| Resuscitation from SCD | 2 (2.1) | 1 (2.2) | .986 |
| SCD or SCD equivalent | 16 (17.0) | 8 (17.4) | .895 |
| Without ICD | Men (n=519) | Women (n=383) | P |
|---|---|---|---|
| Cardiovascular death | 9 (1.7) | 20 (5.2) | .003 |
| Heart failure related death | 4 (0.8) | 9 (2.3) | .046 |
| Myocardial infarction related death | 2 (0.4) | 2 (0.5) | .760 |
| Stroke related death | 1 (0.2) | 2 (0.5) | .396 |
| SCD | 2 (0.4) | 7 (1.8) | .031 |
ICD, implantable cardiac defibrillator; SCD, sudden cardiac death.
Data are expressed as No. (%).
If we analyze the occurrence of SCD stratified by HCM Risk-SCD score, no patients of either sex experienced SCD when in the low risk category. SCD occurred in 1 woman (1/18, 5.6%) and 1 man (1/30, 3.0%), in the intermediate risk category (P=.71), and occurred in 3 women (3/20, 15.0%) and 2 men (2/33, 6.0%) in the high-risk category (P=.28).
DiscussionIn this multicenter HCM registry, women had a worse prognosis with higher overall and CV mortality. Female sex was an independent predictor of mortality in the multivariate analysis. Women had a higher frequency of HF-related death. Despite having a similar SCD risk to men, women underwent less ICD implantation.
In studies on HCM, men are included more frequently than women,7,8,12 but it is currently unknown whether HCM reflects genetic and hormonal modifiers, which make men more vulnerable to disease penetrance, or a bias in screening/diagnostic strategies leading to underdiagnosis and/or late diagnosis in women.13 Perez-Sanchez et al.14 reported that male sex was a factor leading to an earlier diagnosis in patients with sarcomeric mutations, but found no difference in prognosis between sexes. More than half of the population included in our registry underwent genetic testing,9 and the testing results were in line with previously published HCM registries.15 There was no difference between the sexes regarding the number of tests performed or in the results of testing.9 The same applies to active phenocopy exclusion.8,9 In 2005, Olivotto et al.12 showed a 3:2 prevalence of male over female sex in HCM. Men were more often diagnosed fortuitously by routine medical examination than women (41% vs 23%), in whom the diagnosis was established later (38±18 vs 47±23 years), mainly after onset of worse symptoms (NYHA class 1.8±0.8 vs 1.4±0.6). These findings were corroborated by further studies,14,16 and recently Geske JB et al.7 drew the same conclusions in a cohort of 3673 American patients–only 45.2% were women and they were diagnosed at a more symptomatic and advanced stage of the disease. According to these results, we can hypothesize that women may be underrepresented in HCM cohorts due to lower awareness of CV disease in women,17,18 less participation in screening programs, which are mainly driven in young men athletes,19 less often seeking medical help when there are symptoms,13 and less willingness among clinicians to perform diagnostic procedures in women, a phenomenon that has been reported in other CV diseases. 13 Moreover, the consistent reporting of male sex predominance in HCM cohorts and the media and social attention to HCM as an underrecognized major cause of SCD in young male athletes18,20 may have concurred to increased awareness of HCM in men, but not in women, and to the misconception among clinicians of a more severe form of disease in men.
Women more commonly presented with HF, as well as diastolic dysfunction, mitral regurgitation, worse B-type natriuretic peptide levels, and a trend to higher maximum gradient on the LVOT. Geske et al.7 reported that women have more obstructive physiology, which is in line with our findings, and HF in HCM has been reported to be more common in patients with obstructive disease.21 HF with diastolic dysfunction is known to be more prevalent in women22 and it is also related to hypertension,23 which was also more common in women in this registry. This fact can explain the worse diastolic function in women, despite higher LV maximum wall thickness in men. Furthermore, the prevalence of LVOT obstruction in women may be underestimated in our registry, as stress echocardiography was less often performed, which is also a sign for the existence of a gender bias.
Our study found higher overall and CV mortality in women, associated with a significantly higher frequency of HF-related mortality. Despite current articles stating a lower incidence of HF-related death in HCM patients, we identified an overall rate of HF-related death of 2.6% in women. 3 According to Melacini et al.24 HF symptoms in HCM progress along 3 main pathways: a) LV systolic dysfunction, b) LVOT obstruction, and c) the absence of obstruction with preserved systolic function. In this third subgroup of patients, HF symptoms were mainly due to diastolic dysfunction and a more accelerated progression to advanced HF and adverse outcome was identified.21 Interestingly, patients with progressive HF were more commonly women. Therefore, the higher prevalence of HF and diastolic dysfunction may explain the higher overall CV mortality and specifically the higher HF-related death that was found in women in the HCM registry. Sex hormones can also modulate the HCM phenotype, as in other CV diseases.13,25 Bhupathy et al.26 demonstrated increased mortality in phyto-oestrogen-fed male mice in an HCM model, which suggests a possible deleterious effect of oestrogen in HCM. Olivotto et al.12 showed, in a longitudinal trial, that female sex was independently associated with progression to NYHA functional classes III/IV or death from HF or stroke. This was also seen in our results in the overall population. Recently, Geske et al.7 demonstrated that American women with HCM have worse survival than men, a relationship which remained significant on multivariate analyses. Our study corroborates these findings, but extends the analysis showing that overall and CV deaths were also higher in European HCM women and that female sex remained an independent predictor of overall and CV mortality in HCM after multivariate modelling. Despite being a nationwide registry, the results have an international impact, as several countries worldwide share similar conditions in the management of this disease.8
The HCM Risk-SCD score adequately identified all patients with low risk, independently of sex. Despite the same overall SCD risk according both to classic and nonclassic risk factors and the current HCM Risk-SCD score,1,27 there was a statistically significant difference toward less ICD implantation in women. Other registries also demonstrate that men have higher overall ICD implantation rates,28 which is usually explained by higher rates of cardiac ischemic disease. However, when we analyzed patients with HCM with a clinical indication for ICD, either due to the presence of 1 or more classic risk factors or to an HCM Risk-SCD score ≥ 4%, women seemed to receive less ICD than men–and this is, to our knowledge, the first study demonstrating this factor. Although women were overall older, the frequency of comorbidities was not significantly different, which could have justified different implantation rates between sexes (table 1, and ). Women were older at presentation, and age diminishes the impact of some risk factors, such as VT (since the risk conferred by these factors is inversely proportional to age), but the HCM Risk-SCD score includes this variable and still does not justify a lower implantation rate in women.
Therefore, we speculate that ICD implantation is less frequently recommended in women for the same reasons previously hypothesized for their underrepresentation in HCM cohorts, leading clinicians to be less aware of SCD risk and disease severity in women. A trial with a stronger statistical power specifically designed to address this issue should be performed in order to clarify if differences in the ICD implantation rate are also identified.
Studies on dilated cardiomyopathy have also shown that women are underrepresented29 and are less likely to receive an ICD compared with men, despite being eligible.30 Strikingly, the follow-up of HCM patients showed that women without ICD had more SCD than men without an ICD. Taken together, the undertreatment with ICD, despite similar SCD risk, and the higher frequency of SCD in women without ICD suggest that the underimplantation of ICD has a negative prognostic impact in women. These results emphasize that women must be candidates to receive equal prevention strategies, according to SCD risk, as men. The prevalence of SCD in women if the same SCD prevention strategy had been offered to both sexes remains to be determined.
Study limitationsThis study has some limitations. The study may provide a biased perspective of the HCM population, because, of the 29 centers that included patients, 3 included more than 100 patients each. Differences between patients included per center have already been analyzed in a previously published trial: high-volume (> 100 patients, n=3) centers had younger patients, more familial HCM and performed more genetic testing, family screening and exclusion of phenocopies than low-volume centers (< 15 patients included, n=16). However, no major differences in outcomes was found between centers8.
This study includes mostly symptomatic patients, who received medical attention. The frequency of SCD may also be underestimated, as patients who died before the disease diagnosis were not included. These factors may have led to a survival bias, since forms of HCM whose first major manifestation was SCD were not included in the registry. Regarding implanted devices, the reasons for pacemaker implantation were not registered, but the number of pacemaker implants was similar between the sexes. When patients did not undergo ICD implantation, there was no compulsory question regarding whether it was not proposed or whether it was refused by the patient. Risk factors do not confer a static risk over time, and when analyzing the SCD risk profile with classic risk factors, re-evaluation over time for each patient was not available in the registry. Hence, the time exposed to each risk factor and variability in risk during the patient's life could not be included in our analysis, which may alter our perception of the true population at risk at each time point. In addition, we could not calculate the HCM risk score for the entire population included, because not all variables were simultaneously available for all patients.
We were unable to obtain a prospective analysis of patients throughout the years and their respective maximum NYHA class. Patients at older ages are infrequently in NYHA I class without necessarily being in an aggravated HF state, which may lead to HF overdiagnosis.
ConclusionsIn this nationwide HCM registry, women showed clinical, therapeutic and prognostic differences. They were older and more symptomatic than men and their prognosis was worse, with higher CV and overall mortality and more HF-related deaths. On multivariate modelling, female sex remained independently associated with overall and CV mortality. Despite having a similar SCD risk to men, women underwent less ICD implantation. These data highlight the need for an improved clinical approach in women with HCM, with increased awareness of the disease, targeted treatment of HF, and appropriate and timely ICD implantation according to SCD risk stratification.
- -
Hypertrophic cardiomyopathy (HCM) is an inherited cardiac condition, frequently transmitted as an autosomal dominant trait but with a reported small male preponderance.
- -
In 2017 it was reported that, in an American-based population, women seemed to have a worse prognosis, possibly related to gender bias.
- -
This study demonstrates that female sex was independently associated with a worse prognosis in HCM patients, regarding overall and cardiovascular mortality.
- -
Women underwent fewer stress tests and received fewer ICDs, despite having a similar sudden cardiac death risk to men.
- -
The results highlight the need to change the current clinical approach in women with HCM, focusing on increased awareness of the disease, targeted treatment of HF, and appropriate and timely ICD implantation according to SCD risk stratification.
There are no conflicts of interest to declare.
The authors would like to thank J. Nicolas Lopez-Canoa for his assistance with language help and proofreading of the manuscript.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.01.007