Keywords
INTRODUCTION
Ectopic foci that induce paroxysmal atrial fibrillation (PAF) are generally located in the pulmonary veins (PVs).1-4 Nevertheless, several studies have described foci elsewhere, mainly in the posterior wall of the left atrium5 (LA) and in the superior vena cava (SVC).5,6 These sites, although less common, can be clinically relevant in specific cases. We describe our experience in three patients who showed focal activity in the SVC. Two of them were cured with radiofrequency ablation in this vein alone, whereas the third additionally required ablation at the left superior PV in a second procedure.
PATIENTS AND RESULTS
Case 1
A 61-year-old man, who had been paraplegic since his youth due to an accident, had experienced two prior episodes of heart failure associated with sustained atrial tachyarrhythmia (atrial fibrillation and atrial flutter). The cardiologic study was normal, with the exception of mild left ventricular systolic dysfunction. Thyroid function was normal. He came to the emergency room with pulmonary edema, atrial fibrillation (AF), and a rapid ventricular rate. Monitoring during hospitalization showed sinus rhythm with runs of self-limited atrial tachyarrhythmia and episodes of sustained AF. Transesophageal echocardiography confirmed mild left ventricular systolic dysfunction, slight dilation of the LA, and an image consistent with thrombus in the left atrial appendage.
Following 6 weeks of anticoagulation therapy and after confirming resolution of the LA thrombus, an electrophysiological study was performed. A 24-pole catheter was advanced through the femoral vein to simultaneously record the septal and anterolateral aspects of the right atrium (RA), and a deflectable catheter was inserted in the pulmonary artery (PA) for indirect mapping of the LA roof. A multipolar catheter was advanced through the right basilic vein toward the coronary sinus (CS) (Figure 1). Although no ectopic activity was present at baseline, infusion of high doses of isoproterenol induced atrial extrasystoles (AEs), with early activation in the posterior RA roof and late activation in the PA. Insertion of an explorer catheter in the SVC triggered frequent AEs and numerous runs of nonsustained atrial tachycardia. Recordings from the explorer catheter in the SVC at 2 cm above the junction with the RA showed earliest activation during the extrasystoles (Figure 2). After ruling out phrenic nerve stimulation, three applications of radiofrequency energy to the anterior aspect of the SVC were carried out, and the patient had no further episodes of spontaneous arrhythmia or inducible arrhythmia with stimulation maneuvers. Contrast venography showed no significant alterations. Echocardiography performed at one year showed normal left ventricular systolic function. After four years of follow-up, the patient has had no clinical arrhythmias.
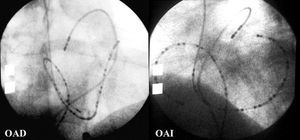
Figure 1. Right anterior oblique (RAO) and left anterior oblique (LAO) radiological views of the reference catheters for mapping of ectopic activity (24-pole catheter in the right atrium, multipolar catheter in the coronary sinus, and deflectable tetrapolar catheter in the pulmonary artery).
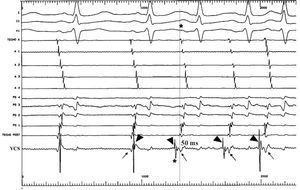
Figure 2. Tracing at initiation of nonsustained atrial tachycardia (case 1). Leads I, II, and V1 are shown, as well as intra-atrial tracings in the anterior wall of the right atrium arranged from top to bottom, tracings in the septal wall of the right atrium arranged from bottom to top, and an explorer recording in the superior vena cava (SVC). During sinus rhythm, the SVC tracing showed two components, the first with lower amplitude (arrows), which coincides with the recording of the P roof, and the second with higher amplitude (arrowheads), which is more delayed. At the start of the arrhythmia (*) the SVC recording shows earlier activity, with inversion in time of the two components of the electrogram (arrowheads); the first deflection precedes the start of the surface P by 50 ms.
Case 2
A 40 year-old woman, smoker with no other factors of interest, had experienced frequent, self-limited palpitations of short duration over the previous year; on two occasions PAF had been documented in the emergency room. The physical examination and echocardiography findings were normal. Holter monitoring with propafenone administration showed high-density AEs with numerous runs of self-limited AF.
The electrophysiological study was aimed at mapping possible focal activity. A 24-pole catheter was inserted to simultaneously record the anterior and septal RA activity, a decapolar catheter was directed toward the CS, and a deflectable explorer catheter was inserted in the right PA. Atrial stimulation triggered highly reproducible extrasystoles, with early recording in the high septal RA and late electrograms in the LA roof and the CS. During SVC mapping, simple pressure induced an automatic rhythm and extrasystoles, with premature beats occurring 50-60 ms early in the SVC recordings during the AEs. After ruling out phrenic nerve stimulation, two applications of radiofrequency energy were carried out, guided by the premature activation in the anterior area of the SVC at 2 cm above the atriocaval junction, thereby suppressing the automatic activity. After a follow-up of 17 months without antiarrhythmia medication, the patient has had no other episodes of arrhythmia, although she sometimes experiences sporadic precordial "jumps" and Holter monitoring shows an occasional AE.
Case 3
A 49-year-old woman with a history of hyperthyroidism-associated PAF in 1996. In 1999, her thyroid function was normal, but the symptoms persisted, with multiple episodes of PAF while she was receiving propafenone treatment. In 2002, ablation of the vena cava-tricuspid isthmus was performed for flutter occurring with the same treatment, but the PAF attacks continued, as well as numerous episodes of self-limited AF and frequent AEs on the Holter recordings.
The electrophysiological study was aimed at locating arrhythmogenic foci. A 24-pole catheter was advanced toward the RA for simultaneous recording of the anterior wall and septal wall, a decapolar catheter was advanced to the CS and an explorer catheter to the PA. Following administration of isoproterenol, AE was induced with early activation in the high RA septum, which triggered nonsustained AF. The earliest activation during extrasystole was located in the SVC; an origin in the PV was ruled out by mapping through the patent foramen ovale. Guided by a Lasso catheter (Cordis Webster, Diamond Bord, CA) placed in the SVC, three applications of radiofrequency energy were performed in the anterior and medial area, thereby electrically excluding the vein (Figure 3). Persistent blockage of the isthmus was confirmed.
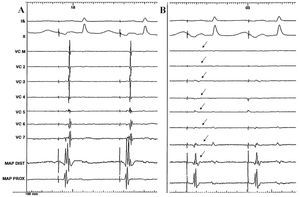
Figure 3. Simultaneous recording (case 3) of the surface leads, bipolar electrograms of the Lasso catheter in the SVC, and explorer catheter in the junction of the SVC and the RA, before ablation. A: stimulated rhythm from the low anterior aspect of the RA. B: note that following radiofrequency application, the potentials recorded in the interior of the vein disappear (arrows) and the second component of the electrogram in the distal pole of the explorer is lost, whereas the recording in the proximal electrode remains unchanged. RA indicates right atrium; MAP DIST, mapping of the distal catheter pole; MAP PROX, mapping of the proximal catheter pole; SVC, superior vena cava.
At 2 months, PAF recurred. A new study confirmed isolation of the SVC and disclosed pronounced ectopic activity originating in the left superior PV that triggered nonsustained AF. The vein was electrically isolated using a Lasso catheter as a guide, and radiofrequency energy was applied with an irrigated catheter. The patient did not wish to discontinue propafenone treatment, and, after 22 months of follow-up, showed no clinical recurrence or alterations on Holter monitoring.
DISCUSSION
The role of the SVC in the genesis of AF was demonstrated in the two cases in which direct ablation of the arrhythmogenic foci completely resolved the arrhythmia. The third patient appeared to have multifocal ectopy because electrical exclusion of a PV in a second procedure was required to resolve the arrhythmia, despite the fact that PVs were initially ruled out as the origin of the ectopy by direct recording through the patent foramen ovale in the first study. This incidence accounts for 14% of all patients undergoing mapping of arrhythmogenic foci prior to ablation in our department and is close to reported values in the literature, which have described foci exclusively in the SVC in 6%5,6 and simultaneously in the SVC and other locations in up to 11%.6 Myocardial extensions into the SVC are the probable source of such ectopic activity,7 which contributes to the initiation and maintenance of AF,5,6 in all likelihood by a mechanism of abnormal automatic activity or a focal triggering activity,8 without ruling out possible reentry mechanisms within the SVC.9
This incidence, although small, indicates the importance of mapping the origin of AEs whether they occur spontaneously or are induced during the study, particularly in recurrent cases following PV ablation. When the P-wave is visible, the ECG can help locate the site of origin. However, when the ectopic P-wave is superimposed on the T-wave and AF is induced with the first AE, study of the P-wave becomes more difficult. The location in the SVC has the added importance of easy access, making trans-septal catheterization unnecessary. In this context, a catheter in the CS and another in the PA to record activity in the LA roof are helpful for ruling out ectopic foci in the PV or the LA when ECG recordings in these locations are delayed with respect to those of the RA.
Induction of repetitive extrasystoles and runs of AF when the explorer catheter was inserted in the SVC was a noteworthy finding, particularly in case 1, in which stimulation and isoproterenol did not induce arrhythmia. It is difficult to separate this finding from a mere artifact in each individual case, but perhaps it should be regarded as important when no spontaneous or inducible ectopic activity from other origins is detected.
Correspondence: Dr. D.A. Pastor.
Servicio de Cardiología. Hospital Universitario de Getafe.
Ctra. de Toledo, km 12.5. 28905 Getafe. Madrid. España.
E-mail: apastor@hugf.insalud.es
Received September 5, 2005.
Accepted for publication March 9, 2006.