More than 40 million people are vaccinated against COVID-19 in Spain.1 Adverse reactions to the vaccine are usually insignificant and do not outweigh the benefits. In relation to cardiac adverse effects, complete heart block (CHB) was not reported in the clinical trials of COVID-19 vaccines.2
We report a case of CHB with temporal association with COVID-19 vaccine administration, which recovered with corticotherapy. Written informed consent for publication was obtained from the patient.
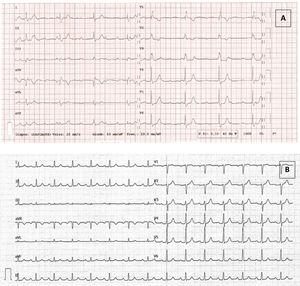
Six days after the first dose of SARS-CoV-2 mRNA vaccine (Pfizer-BioNtech, United States), a 49-year-old man presented to the emergency department with dizziness and dyspnea, with onset 3 days previously. Physical examination revealed bradycardia. Blood pressure was 136/60 mmHg, heart rate 40 beats/min, oxygen saturation was 100% and the patient was afebrile. Electrocardiogram (ECG) showed CHB with right bundle branch block (figure 1A). A blood test showed normal renal function, electrolytes and hemogram. C-reactive protein (CRP) was 15.7 mg/L (< 5), high-sensitivity troponin T 17 ng/L (< 13), creatine kinase 57 U/L (< 189), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) 307 ng/L (< 300). Transthoracic echocardiography showed normal ejection fraction without structural heart disease.
The patient had had a nonseminomatous testicular germ cell tumor in 2003 with pulmonary metastatic disease. He was treated with orchiectomy and chemotherapy with complete remission. His previous ECG was normal.
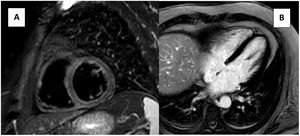
During hospitalization, a blood test showed normal electrolyte concentration and minor CRP elevation (15.7→11.7 mg/L). Negative troponin (17→17→12 ng/L) and the absence of ventricle wall motion abnormalities ruled out ischemic heart block (HB). Transthoracic echocardiography and cardiac magnetic resonance (CMR), with a protocol including cine, short tau inversion recovery (STIR), T1, T2 mapping and late gadolinium enhancement sequences, revealed normal cardiac function and structure and absence of edema, excluding cardiomyopathies or myocarditis (figure 2). Negative immunological study (ANA, ENA) and serologies (Borrelia burgdorferi, cytomegalovirus, Epstein-Barr, Hepatitis A, B, C, herpes simplex, measles, Treponema pallidum, Toxoplasma gondii, varicella-zoster, HIV, rubella, and mumps virus) excluded autoimmune and infectious diseases. A previous normal ECG excluded congenital HB.
The patient was admitted under the diagnosis of CHB. A comprehensive etiological study found no abnormalities. Since he had been recently vaccinated, CHB caused by local inflammation of the conduction system associated with the vaccine was suspected. Corticotherapy with prednisone 1 mg/kg per day was started, and, after 2 doses, the patient experienced resolution of CHB. Afterwards there was a progressive improvement of conduction, with progressive shortening of the PR interval and QRS until complete normalization (figure 1B).
After 16 days, the patient was discharged. After a month, the ECG remained normal, and prednisone was slowly reduced until discontinuation. SARS-CoV-2 serology showed immunity after the COVID19 vaccine.
We describe a patient who presented with CHB after Pfizer/BioNTech mRNA COVID-19 vaccination. Previous atrioventricular (AV) conduction could be proved normal. Cardiac disorders were highly infrequent in COVID-19 vaccine clinical trials. With the Pfizer/BioNTech mRNA COVID-19 vaccine, less than 0.1% of participants experienced a cardiac event.2 CHB were not reported in any clinical trials of COVID-19 vaccines.
Recently, a case series study observed an increased risk of cardiac arrhythmias, including HB, following a second dose of the mRNA COVID-19 vaccine and in the first 28 days following a SARS-CoV-2 positive test.3
The differential diagnosis included myocarditis, as there is a plausible causal relationship between myocarditis and mRNA vaccines.4 However, in case series describing myocarditis following COVID-19 vaccination, all patients presented with acute chest pain, significantly elevated troponin levels, and compatible CMR findings.
Inflammation and fibrosis can play important role in conduction disturbances. When inflammation extends to AV node, it may cause CHB.8 F-fluoro-deoxy-glucose positron emission tomography/computed tomography (PET/CT) has a potential role in this scenario.5 Corticotherapy is known to improve AV conduction in some situations, such as HB in cardiac sarcoidosis. Patients who benefit from corticotherapy are those with septal inflammation affecting AV conduction, but with no or minimal fibrosis.
Although we could not demonstrate inflammation, we speculated that localized inflammation of the conduction system associated with an inflammatory response to vaccination caused CHB and started empiric corticotherapy (1mg/kg/d), with rapid improvement of cardiac conduction. There was no fibrosis on CMR, which could explain the recovery. Standard prednisone tapering was done (reduction of 10 mg every 5 days).
Hitherto, only isolated clinical cases have reported an association between HB and SARS-CoV-2 vaccination, mostly in elderly patients with underlying conduction disorders. Nasab et al.6 published a case report of a 65-year-old patient without previous cardiac disease who developed 2:1 AV block a few days after COVID-19 vaccination and required permanent pacemaker implantation.
Our case is the first to show a CHB in a young patient without pre-existing conduction disease and evidence of resolution of the conduction disorder with corticotherapy. Whether HB was related to an excessive inflammatory response to the vaccine remains unknown and the use of alternative anti-inflammatory therapies needs further investigation.
In this case, we report a patient with CHB with a consistent temporal association with COVID-19 vaccine administration, who recovered normal AV conduction after 4 weeks of corticotherapy. Although the etiology of the HB is unknown, the clinical course and effect of the corticosteroid suggests inflammation of the conduction system due to an inflammatory response to vaccination. CHB as a possible vaccine-related adverse event is a finding that requires further study.
FUNDINGThe authors received no financial support for the research, authorship and/or publication of this article.
AUTHORS’ CONTRIBUTIONSConceptualization: P. Mañas; writing - original draft: A. Pons-Riverola; writing - review and editing: A. Pons-Riverola, P. Mañas, E. Claver, O. Meroño, J. Comín-Colet, I. Anguera; supervision: P. Mañas, J. Comín-Colet, and I. Anguera.
CONFLICTS OF INTERESTThe authors declare that there is no conflict of interest.