There are limited data on the long-term development of neoaortic root dilatation (NRD) and neoaortic valve regurgitation (AR) after arterial switch operation (ASO) for transposition of the great arteries during adult life.
MethodsWe performed a retrospective longitudinal analysis of 152 patients older than 15 years who underwent ASO for transposition of the great arteries and who were followed-up for 4.9±3.3 years in 2 referral centers. Sequential changes in body surface-adjusted aortic root dimensions and progression to moderate/severe AR were determined in patients with 2 or more echocardiographic examinations. Risk factors for dilatation were tested by Cox regression to identify predictors of AR progression.
ResultsAt baseline, moderate AR was present in 9 patients (5.9%) and severe AR in 4 (2.6%), of whom 3 had required aortic valve surgery. Initially, the median neoaortic root dimension was 20.05±2.4mm/m2, which increased significantly to 20.73±2.8mm/m2 (P <.001) at the end of follow-up. The mean change over time was 0.14mm/m2/y (95%CI, 0.07-0.2). Progressive AR was observed in 20 patients (13.5%) and 6 patients (4%) required aortic valve surgery. Progressive AR was associated with bicuspid valve, AR at baseline, NRD at baseline, and neoaortic root enlargement. Independent predictors were bicuspid valve (HR, 3.3; 95%CI, 1.1-15.2; P=.037), AR at baseline (HR, 5.9; 95%CI, 1.6-59.2; P=.006) and increase in NRD (HR, 4.1 95%CI, 2-13.5; P=.023).
ConclusionsIn adult life, NRD and AR progress over time after ASO. Predictors of progressive AR are bicuspid valve, AR at baseline, and increase in NRD.
Keywords
Transposition of the great arteries (TGA) is the most common cyanotic heart disease present at birth. Several techniques exist for repairing this defect, but the current surgical procedure of choice is the arterial switch operation (ASO). This is an anatomic repair technique performed in the neonatal period. It has the advantage of maintaining the morphologic left ventricle as a systemic ventricle, although embryologically the functional unit comprising the neoaortic root (valve, annulus, and sinus portion) corresponds to the pulmonary valve and artery.1
Neoaortic root dilatation (NRD) and neoaortic valve regurgitation (AR) are 2 of the most serious complications of ASO. ASO outcomes in childhood are well known,2,3 and although many pediatric patients experience progressive dilatation of the ascending aorta and AR, these are mostly mild and few cases (1%-3%) require surgical repair.3
The number of young adults who underwent ASO and now require follow-up at an adult congenital heart disease (ACHD) unit has been increasing for several years now. Few studies, however, have analyzed the occurrence or progression of NRD or AR in this population and those that have done so have reported contrasting results, with some studies showing favorable outcomes and stabilization of NRD4 and AR,5,6 and others showing significant progression and a high number of reinterventions.7,8
The aims of this study were to describe the prevalence of significant AR in young adults in ACHD care and the need for reintervention due to aortic complications and incidence of serious clinical events in this population. Additional aims were to investigate the presence of progressive NRD and AR and to identify predictors of AR progression.
MethodsStudy populationRetrospective, longitudinal analysis of a cohort of patients at 2 referral ACHD units who had undergone ASO for TGA or Taussig-Bing anomaly. Patients were included at the time of transfer to the ACHD unit and inclusion criteria were a follow-time of over 1 year and an echocardiographic evaluation at baseline and annually thereafter. The study was conducted in accordance with the principles of the Declaration of Helsinki and patients were included in the Spanish National ACHD Registry (RECCA). The study was approved by the ethics committees at the 2 participating hospitals (study 2017/0659).
Study variablesVariables related to structural abnormalities, initial surgery, and clinical course during pediatric care were collected retrospectively. Study variables included demographic characteristics, type of TGA, associated anomalies (ventricular septal defect [VSD], coarctation of the aorta, left ventricular outflow tract [LVOT] obstruction, native bicuspid pulmonary valve), coronary pattern (classified as normal, right and left arising in the same sinus, intramural, and other), ASO variables (age at time of operation and previous pulmonary artery banding), and need for surgical or percutaneous reinterventions).
The variables collected for the follow-up period in the ACHD units were age at first and last visit, neoaortic root diameter (sinus portion) indexed by body surface area, degree of AR, and clinical events. Clinical events included death, severe coronary events (death, myocardial infarction, and coronary revascularization), arrhythmias (ventricular tachycardia and atrial fibrillation or flutter), endocarditis, and surgical or percutaneous reinterventions.
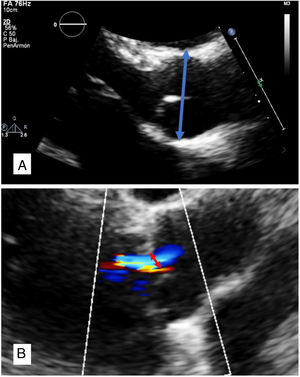
To standardize the results, neoaortic root variables were measured at both ACHD units exclusively by transthoracic echocardiography. The aorta was measured at the level of the sinuses of Valsalva, at end-diastole, and from leading edge to leading edge (figure 1A). NRD was defined as a neoaortic root diameter of at least 20.6mm/m2 in men and 20.7mm/m2 in women. This value was calculated by adding the mean plus 1 standard deviation (SD) of the dimensions of the sinus portion of the aorta indexed by body surface area and sex established by Saura et al.9 as normal in a cohort of controls. Growth was defined as the difference between the baseline measurement (first visit to ACHD unit) and the final measurement (last visit to ACDH unit) indexed by body surface area and divided by years of follow-up (mm/m2/y).
AR was studied by analyzing the vena contract using color Doppler echocardiography. Degree of AR was semiquantitatively classified as mild (<3mm), moderate (3-6mm), or severe (>6mm)10 (figure 1B). Significant AR was defined as moderate and severe regurgitation. AR was considered to have progressed when 2 successive cardiographic evaluations showed progression to moderate or severe AR.
Statistical analysisContinuous variables are expressed as mean±SD for normally distributed data and as median [IQR] for nonnormally distributed data. Qualitative variables are shown as percentages.
Follow-up time was calculated as the time from the first to the last echocardiographic evaluation at the corresponding ACHD unit. Patients without an echocardiographic evaluation after the first year of follow-up were not included in the analyses.
Mean neoaortic root diameter at baseline and end of follow-up was compared using the paired sample t test to confirm the presence of progressive NRD. Mean neoaortic growth was calculated as the difference between the first and last measurements in mm/m2/y. Means of independent variables were compared to identify factors associated with NRD.
Kaplan-Meier survival curves were built to estimate survival free of significant AR and neoaortic root surgery. To analyze AR progression, the date of the echocardiogram showing an increase in AR severity was noted. Patients with severe AR during childhood were excluded from this analysis regardless of whether or not they had undergone surgery. Progression-free survival was also estimated using Kaplan-Meier curves. The individual contribution of different risk factors to AR progression was analyzed using multivariate Cox regression. Statistical significance was set at a P value of less than .05. Statistical analyses were performed using the SPSS 15.0 package for Windows (SPSS Inc., United States).
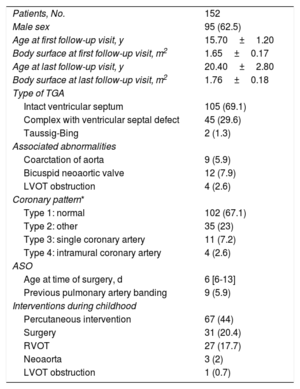
ResultsIn total, 156 patients (62.5% male) who had undergone ASO for TGA were transferred from pediatric cardiology to ACHD care. Mean age at the first visit to the ACHD unit was 15.7±1.2 years; 152 patients had a follow-up time of over 1 year (4 patients were excluded due to loss to follow-up) (figure 2). Mean follow-up was almost 5 years (4.9±3.3 years) and mean age at the end of follow-up was 20.4±2.8 years (figure 3).
Demographic and anatomic characteristics are provided in table 1 together with details of the ASO and any reinterventions performed in pediatric care. Most patients had dextro-TGA with a VSD involving almost a third of the septum; 7.9% of patients had a bicuspid neoaortic valve. Thirty-one patients (20.4%) had undergone surgery during childhood. The most common indication (27 patients) was treatment of the right ventricular outflow tract (RVOT) or pulmonary artery. The other indications were AR (prosthetic implant in 2 patients and valve repair in 1) and LVOT obstruction (1 patient).
Patients’ clinical characteristics
| Patients, No. | 152 |
| Male sex | 95 (62.5) |
| Age at first follow-up visit, y | 15.70±1.20 |
| Body surface at first follow-up visit, m2 | 1.65±0.17 |
| Age at last follow-up visit, y | 20.40±2.80 |
| Body surface at last follow-up visit, m2 | 1.76±0.18 |
| Type of TGA | |
| Intact ventricular septum | 105 (69.1) |
| Complex with ventricular septal defect | 45 (29.6) |
| Taussig-Bing | 2 (1.3) |
| Associated abnormalities | |
| Coarctation of aorta | 9 (5.9) |
| Bicuspid neoaortic valve | 12 (7.9) |
| LVOT obstruction | 4 (2.6) |
| Coronary pattern* | |
| Type 1: normal | 102 (67.1) |
| Type 2: other | 35 (23) |
| Type 3: single coronary artery | 11 (7.2) |
| Type 4: intramural coronary artery | 4 (2.6) |
| ASO | |
| Age at time of surgery, d | 6 [6-13] |
| Previous pulmonary artery banding | 9 (5.9) |
| Interventions during childhood | |
| Percutaneous intervention | 67 (44) |
| Surgery | 31 (20.4) |
| RVOT | 27 (17.7) |
| Neoaorta | 3 (2) |
| LVOT obstruction | 1 (0.7) |
ASO, arterial switch operation; Cx, circumflex artery; LAD, left anterior descending coronary artery; LVOT, left ventricular outflow tract; R, right coronary artery; RVOT, right ventricular outflow tract; TGA, transposition of the great arteries.
Values are expressed as No. (%), mean±standard deviation, or median [interquartile range].
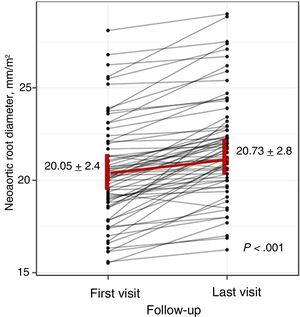
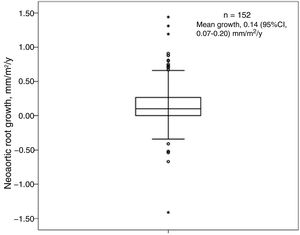
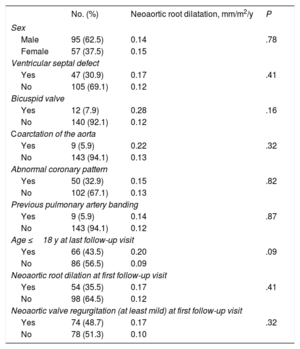
NRD was detected in 38.8% of patients at baseline. Absolute diameter size was > 40mm in 10.5% of patients and > 45mm in 0.6%; none of the patients had a diameter of > 50mm. At the end of follow-up, 48.7% of patients had NRD, with an absolute diameter size of > 40mm in 27.6% of patients, > 45mm in 9% of patients, and > 50mm in just 2 patients. Mean NRD increased both significantly (P<.001) and progressively from 20.05±2.4mm/m2 at baseline to 20.73±2.8mm/m2 at the end of follow-up (figure 3). The mean growth rate was 0.14mm/m2/y (95% confidence interval [95%CI], 0.07-0.2) (figure 4). None of the study variables were significantly associated with progressive NRD (table 2).
Variables associated with neoaortic root dilatation
| No. (%) | Neoaortic root dilatation, mm/m2/y | P | |
|---|---|---|---|
| Sex | |||
| Male | 95 (62.5) | 0.14 | .78 |
| Female | 57 (37.5) | 0.15 | |
| Ventricular septal defect | |||
| Yes | 47 (30.9) | 0.17 | .41 |
| No | 105 (69.1) | 0.12 | |
| Bicuspid valve | |||
| Yes | 12 (7.9) | 0.28 | .16 |
| No | 140 (92.1) | 0.12 | |
| Coarctation of the aorta | |||
| Yes | 9 (5.9) | 0.22 | .32 |
| No | 143 (94.1) | 0.13 | |
| Abnormal coronary pattern | |||
| Yes | 50 (32.9) | 0.15 | .82 |
| No | 102 (67.1) | 0.13 | |
| Previous pulmonary artery banding | |||
| Yes | 9 (5.9) | 0.14 | .87 |
| No | 143 (94.1) | 0.12 | |
| Age ≤18 y at last follow-up visit | |||
| Yes | 66 (43.5) | 0.20 | .09 |
| No | 86 (56.5) | 0.09 | |
| Neoaortic root dilation at first follow-up visit | |||
| Yes | 54 (35.5) | 0.17 | .41 |
| No | 98 (64.5) | 0.12 | |
| Neoaortic valve regurgitation (at least mild) at first follow-up visit | |||
| Yes | 74 (48.7) | 0.17 | .32 |
| No | 78 (51.3) | 0.10 | |
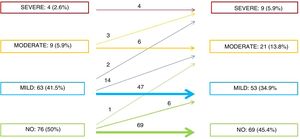
Four patients (2.6%) had severe AR as children and 3 of them had undergone surgery. The prevalence rates for moderate and mild AR at baseline were 5.9% and 41.5%, respectively.
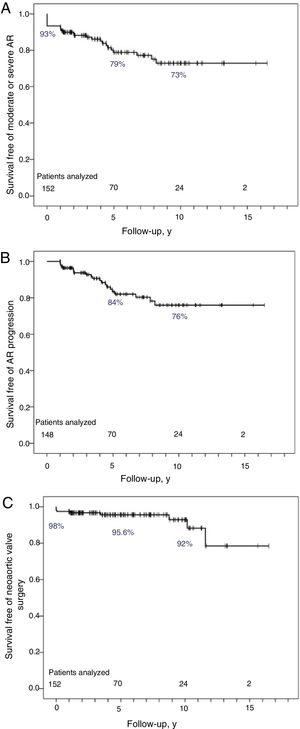
AR became more severe during ¡follow-up in 20 patients (13.5%); 22% of the patients with mild AR at baseline developed moderate AR while 2 patients developed severe AR. Just 1 patient without AR at baseline (1.3%) experienced worsening (to moderate AR). The rates of AR progression and the severity of this condition at the end of follow-up are summarized in figure 5. More than 50% of patients had some degree of AR, and while it was usually mild, almost 20% of patients had significant AR or required surgery. Survival free of moderate or severe AR was 79% at 5 years and 73% at 10 years (figure 6A).
Kaplan-Meier survival curves for patients analyzed during follow-up. A, Survival free of significant (moderate or severe) AR. B: survival free of progression to moderate or severe AR in 148 patients (4 patients with severe AR at baseline were excluded). C: survival free of surgery of the sinus portion of the neoaortic root and the neoaortic valve. gr6 AR, neoaortic valve regurgitation.
In the subanalysis of 148 patients without severe AR at baseline, progression-free survival was 84% at 5 years and 76% at 10 years (figure 6B). Particularly noteworthy was the reduction in the incidence of AR progression after the 5-year point (when patients were approximately 20 years old).
During follow-up, 6 patients, all with severe AR, underwent neoaortic valve surgery; 5 were symptomatic and 1 had left ventricular systolic dysfunction. None of the patients underwent surgery to treat NRD only. Four patients required mechanical aortic valve implantation (combined with mitral valve surgery in 1 patient) and 2 neoaortic valve repairs (combined with aortic valvuloplasty in 1 case). Five- and 10-year neoaortic valve surgeryffree survival rates were 95.6% and 92%, respectively (figure 6C).
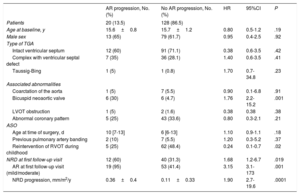
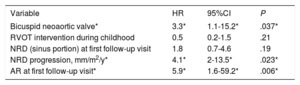
Factors associated with AR progression were a bicuspid valve, baseline AR, baseline NRD, and neoaortic root growth (progressive NRD). Patients requiring surgery due to RVOT complications during pediatric care experienced lower rates of AR progression (table 3). Independent predictors of AR progression (table 4) were a bicuspid valve (hazard ratio [HR], 3.3; 95%CI, 1.1-15.2; P=.037), baseline AR (HR, 5.9; 95%CI, 1.6-59.2; P=.006), and neoaortic root growth (HR, 4.1; 95%CI, 2-13.5; P=.023).
Comparison of baseline characteristics and NRD in patients according to the presence or absence of AR: univariate Cox regression
| AR progression, No. (%) | No AR progression, No. (%) | HR | 95%CI | P | |
|---|---|---|---|---|---|
| Patients | 20 (13.5) | 128 (86.5) | |||
| Age at baseline, y | 15.6±0.8 | 15.7±1.2 | 0.80 | 0.5-1.2 | .19 |
| Male sex | 13 (65) | 79 (61.7) | 0.95 | 0.4-2.5 | .92 |
| Type of TGA | |||||
| Intact ventricular septum | 12 (60) | 91 (71.1) | 0.38 | 0.6-3.5 | .42 |
| Complex with ventricular septal defect | 7 (35) | 36 (28.1) | 1.40 | 0.6-3.5 | .41 |
| Taussig-Bing | 1 (5) | 1 (0.8) | 1.70 | 0.7-34.8 | .23 |
| Associated abnormalities | |||||
| Coarctation of the aorta | 1 (5) | 7 (5.5) | 0.90 | 0.1-6.8 | .91 |
| Bicuspid neoaortic valve | 6 (30) | 6 (4.7) | 1.76 | 2.2-15.2 | .001 |
| LVOT obstruction | 1 (5) | 2 (1.6) | 0.38 | 0.38 | .38 |
| Abnormal coronary pattern | 5 (25) | 43 (33.6) | 0.80 | 0.3-2.1 | .21 |
| ASO | |||||
| Age at time of surgery, d | 10 [7-13] | 6 [6-13] | 1.10 | 0.9-1.1 | .18 |
| Previous pulmonary artery banding | 2 (10) | 7 (5.5) | 1.20 | 0.3-5.2 | .37 |
| Reintervention of RVOT during childhood | 5 (25) | 62 (48.4) | 0.24 | 0.1-0.7 | .02 |
| NRD at first follow-up visit | 12 (60) | 40 (31.3) | 1.68 | 1.2-6.7 | .019 |
| AR at first follow-up visit (mild/moderate) | 19 (95) | 53 (41.4) | 3.15 | 3.1-173 | .001 |
| NRD progression, mm/m2/y | 0.36±0.4 | 0.11±0.33 | 1.90 | 2.7-19.6 | .0001 |
95%CI, 95% confidence interval; AR, neoaortic valve regurgitation; ASO, arterial switch operation; HR, hazard ratio; LVOT, left ventricular outflow tract; NRD, neoaortic root dilatation; RVOT, right ventricular outflow tract; TGA, transposition of the great arteries.
Values are expressed as No. (%), mean±standard deviation, or median [interquartile range].
Predictors of AR progression: multivariate Cox regression
| Variable | HR | 95%CI | P |
|---|---|---|---|
| Bicuspid neoaortic valve* | 3.3* | 1.1-15.2* | .037* |
| RVOT intervention during childhood | 0.5 | 0.2-1.5 | .21 |
| NRD (sinus portion) at first follow-up visit | 1.8 | 0.7-4.6 | .19 |
| NRD progression, mm/m2/y* | 4.1* | 2-13.5* | .023* |
| AR at first follow-up visit* | 5.9* | 1.6-59.2* | .006* |
95%CI, 95% confidence interval; AR, neoaortic valve regurgitation; HR, hazard ratio; RVOT, right ventricular outflow tract; NRD, neoaortic root dilatation.
There was 1 sudden cardiac death during follow-up. Two patients experienced myocardial infarction (both after aortic valve surgery) and there were no reinterventions due to a coronary cause.
Additional events included 2 cases of endocarditis not requiring surgery (both involving the neopulmonary valve) and 4 cases of arrhythmia (2 atrial flutters, 1 supraventricular tachycardia due to nodal re-entry tachycardia, and 1 sustained ventricular tachycardia arising in the right coronary sinus of the neoaortic root that recurred after 2 ablation procedures and required treatment with an implantable defibrillator [the only case in this series]).
Thirteen patients (8.6%) underwent surgical reintervention during follow-up: 6 for AR and 7 for RVOT dysfunction.
Sixteen patients (10.5%) underwent percutaneous coronary intervention. The main cause (14 patients) was pulmonary artery stenosis. In the remaining 2 patients, 1 patient underwent percutaneous pulmonary valve implantation and the other received a stent to treat coarctation of the aorta.
DiscussionASO is the surgical procedure of choice for TGA. It is associated with high mid-term survival, but with time it can result in serious complications such as NRD and AR.3,11
Although ACHD units are seeing increasing numbers of young patients who underwent ASO, few studies have analyzed the incidence of NRD and AR in this population.5–7 In addition, because these patients are still growing, it is difficult to determine whether the changes observed are pathological or due to somatic growth.
In this study, the first of its kind, we analyzed NRD indexed by body surface area in a population of patients still undergoing significant height and weight changes and investigated predictors of AR progression based on the characteristics of patients when first seen at an ACHD unit.
Neoaortic root dilationA range of causes have been proposed to explain NRD, including histomorphologic changes—patients with TGA have been found to have a thinner collagen layer and altered distribution12—and anatomic changes, with an initial disproportion between the sizes of the aorta and pulmonary artery observed in patients with VSD or Taussig-Bing-like anomalies.4 Additional causes proposed include surgical factors, such as denervation13 and the presence of an excessively acute aortic angle that could alter aortic dynamics and increase stress on the arterial wall.14
Although NRD is a recognized complication of ASO, there is no consensus on its progression. While the Boston group found that dilatation occurred up to 10 years of follow-up and then stabilized,4 other authors have reported progressive growth,15–17 although most of the studies analyzed patients aged 15 years or younger.
Our findings show that patients experienced progressive, significant NRD, quantified as 0.14mm/m2/y (mean growth in the range of 0.23-0.25mm/y). This growth rate is clearly greater than that reported for healthy individuals (0.08mm/y),18 yet is far from the rate described for patients with Marfan syndrome (0.49mm/y)19 or a bicuspid valve (0.42mm/y).19 It is also much lower than the rate observed in patients following the Ross procedure (0.43mm/y).20 Nevertheless, a recent study reported a mean progression rate of 0.63mm/y,17 which is much higher than the rate observed in our series. This difference could be due to age differences at the end of follow-up, as the patients had a median age of 12.2 [IQR, 1-39] years at the last follow-up compared with 19.5 [IQR, 15-34] years in our series. Our results are similar to those published by van der Bom et al.,7 who reported a growth rate of 0.28mm/y in a population older than 17 years. The authors also observed greater NRD progression in younger patients but found that this declined with age. We also observed a tendency toward greater growth of the neoaortic root in younger patients, possibly explaining why just 2 patients in our series had a diameter larger than 50mm at the last follow-up visit and why we observed no cases of aortic rupture or dissection. In addition, none of the patients in our series required surgical treatment based purely on the size of the aneurysm. Studies are needed to define NRD progression in older patients and to investigate the influence of risk factors such as high blood pressure on changes in the neoaortic root.
AR: prevalence, progression, and surgeryAR is a common complication of ASO. In our study, 8.5% of patients had moderate or severe AR when first evaluated by the ACHD unit and 3 required surgery. This rate is consistent with rates of between 5.3% and 9.0% reported for pediatric patients aged 15 years.3,4,21
Progressive AR was confirmed in 13.5% of patients at the last follow-up visit, and the 5- and 10-year rates for survival free of moderate or severe AR were 79% and 73%, respectively; these results are similar to those published by Lo Rito et al.8 and van der Bom et al.7 By contrast, Tobler et al.5 and Kempny et al.,6 on analyzing older patients (mean ages of 21 and 25 years), found no progression in AR severity after the age of 18 years, and while prevalence was high (29%-52%), the degree of valve injury was essentially mild. We observed a decline in AR progression after 5 years of follow-up (ie, in patients aged 20 years), and believe this might be due to the slower growth of the neoaortic root growth observed in older patients by van der Bom et al.7
We identified 3 predictors of AR progression: neoaortic root growth, a bicuspid valve, and the presence of AR (of any severity) at the first visit to the ACHD unit. Although progressive NRD has not been found to be associated with AR progression,7 studies using mechanical models simulating the aortic root have shown that progressive growth of the diameter of the sinotubular junction and sinuses places increasing stress on the aortic cusps, particularly at the aortic side, causing tissue remodeling and thickening and calcification of the free edge of the cusps.22 NRD thus would cause AR via a combined mechanism (insufficient coaptation due to dilation and organic involvement of the cusps). In addition to this combined mechanism, patients with a bicuspid valve frequently experience prolapse or pseudo-prolapse, and while the presence of a bicuspid valve has not been identified as a causative factor in pediatric AR,4–21 a recent study in adults did find it to be an important predictor.14
Finally, AR at baseline identifies patients at risk for progression to significant AR.8,21 In our series, just 1 patient (1.3%) without AR when first seen by the ACHD unit developed AR (of moderate severity).
Survival free of neoaortic valve surgery after 5 and 10 years’ follow-up was 95.6% and 92%, respectively, supporting previous findings.5,6 All the patients who developed severe AR during childhood or young adulthood underwent surgery for clinical symptoms or left ventricular dysfunction. Poor tolerance of this adverse hemodynamic situation might be due to the high incidence of diastolic dysfunction and reduced myocardial contractility (longitudinal strain) described in these patients.23,24
Clinical events during follow-upSurvival rates in young adults after ASO are very good. In our study, there was 1 death and 2 myocardial infarctions and both occurred after neoaortic valve surgery. No other coronary events were reported. This low incidence of clinical events is consistent with the findings of a recent meta-analysis reporting a 1.6% incidence rate for coronary complications25 and supports the current recommendation not to perform stress tests for ischemia in asymptomatic patients.26
The incidence of cardiac reintervention in the young adults in our series was high, at 8.6%, and is similar to rates reported elsewhere.5,6 Contrasting with the indications during childhood, the reasons for reintervention during ACHD care included neoaortic root and neoaortic valve complications and RVOT dysfunction. Nevertheless, the progressive nature of lesions affecting the neoaortic valve and sinus portion of the neoaortic root and the impact of cardiovascular risk factors acquired in adulthood indicate an increased likelihood of surgery due to aortic root complications in adult patients.
LimitationsThis study has limitations inherent to its retrospective design. Nonetheless, follow-up criteria at the ACHD units are based on the recommendations of the guideline for the management of adults with congenital heart disease26 and include an annual clinical and echocardiographic evaluation.
Although echocardiographic studies were performed by experts in congenital heart defects, they were performed in 2 different hospitals and the results were not reassessed using standardized criteria. In addition, the technique was not validated by computed tomography or magnetic resonance imaging.
Neoaortic root growth was measured using the sinus portion of the aorta, without consideration of the annulus or sinotubular junction. AR was assessed using a semiquantitative approach, in line with current recommendations.10 In the statistical analysis, the relatively low number of patients who experienced AR progression (n=20) will have limited the reliability of the multivariate model and the accuracy of the coefficients generated.
ConclusionsNRD and AR progressed over the course of follow-up in young adults with TGA treated with ASO. The presence of a bicuspid valve, AR at baseline, and neoaortic root growth were all predictors of significant AR progression. Studies are needed to determine whether AR continues to progress in older patients and to analyze the influence of risk factors such as hypertension on the occurrence of neoaortic root and valve complications.
Conflicts of InterestP. Gallego is associate editor of Revista Española de Cardiología. The editorial policy at the journal to guarantee impartial processing of this manuscript was followed.
- –
ASO is the surgical procedure of choice for TGA and young adults who have undergone this procedure form a growing segment of patients seen at ACHD units.
- –
NRD is a common complication in pediatric patients, but it is not known whether this condition progresses in young adults who are still undergoing somatic growth changes.
- –
Progressive AR in adult patients is a controversial subject and it is difficult to identify who is at increased risk.
- –
NRD continues to progress in young adults, with a mean estimated growth rate of 0.14mm/m2/y.
- –
AR is a common, progressive complication of ASO in adult patients, although its progresion slows with time.
- –
Neoaortic root growth, a bicuspid valve, and AR at baseline are predictors of AR progression and can be used to identify patients with valve disease who require more frequent follow-up visits.