To the Editor:
Torsades de pointes (TdP) tachycardia is a rare polymorphic ventricular tachycardia characterized by short, self-limiting runs, and a prolonged QT interval, which can deteriorate into persistent ventricular fibrillation and sudden death. This arrhythmia is gaining importance, since it is known to be related to commonly prescribed drugs and electrolyte abnormalities.
Most of the drugs that induce TdP can increase both the absolute QT and the corrected QT (QTc). Antihistamines are among the drugs that produce QT prolongation.1,2 Abnormalities of the repolarization reserve are not evident at baseline and only become apparent when the subject is exposed to QT-prolonging drugs or other risk factors.3
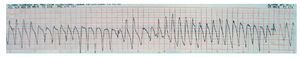
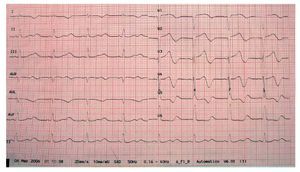
We describe a 73-year-old man with a history of diabetes, dyslipidemia, intermittent claudication, and adenocarcinoma of the prostate. The patient had been taking rupatadine (10 mg/day) for the last week for cold symptoms. He consulted for presyncopal episodes over the last 4 days accompanied by sweating, and dizziness, along with a syncopal episode that resolved within a few seconds. During the patient's stay in the emergency room, he experienced another syncopal episode, with polymorphic ventricular tachycardia due to TdP (Figure 1) that required electrical cardioversion on 2 occasions and intravenous magnesium infusion for subsequent control. He also presented a single recurrence that remitted spontaneously. The ECG showed sinus bradycardia, even though the patient was not taking any bradycardia-inducing drugs, with left bundle-branch block and QT interval prolongation (QTc of 680 ms) and 2-phase T-wave in the precordial leads (Figure 2). The laboratory workup, which included magnesium, thyroid hormones, and troponin, was normal. A left ventricle of normal size, structure, and contractility, and with no valve disease was observed on echocardiography.
Figure 1. Polymorphic ventricular tachycardia due to torsades de pointes.
Figure 2. Corrected QT of 680 ms.
The patient had undergone electroencephalography and computed tomography 6 months earlier in the neurology department to investigate the syncopal symptoms, with normal results. The previous electrocardiograms showed a QTc of 547 ms. The patient was diagnosed with aborted sudden death, caused by TdP secondary to idiopathic long QT syndrome and exacerbated by rupatadine, and was given a cardioverter defibrillator. He was also told to discontinue rupatadine and avoid QT-prolonging drugs, and has remained asymptomatic since that time, without any shocks. After 9 months of follow-up, the current QTc was 460 ms.
Second-generation antihistamines are relatively free of adverse effects. However, some drugs such as terfenadine and astemizole have been withdrawn from the market because of the risk of QT interval prolongation and the possibility of TdP development attributed to these agents. There are doubts about whether this effect can be considered a class effect of all antihistamines or whether it is specific to some molecules. It has been shown that elevated plasma concentrations of these drugs prolong cardiac repolarization due to blockade of cardiac potassium channels that determine the duration of the action potential.4
Rupatadine is a nonsedating, long-acting histamine antagonist with peripheral selective H1-receptor activity.
The rupatadine dose needed to block potassium channels in vitro is 400-fold the dose used in clinical practice and, therefore, no antiarrhythmic effects of this medication are expected.5 The summary of product characteristics for this drug does not include cardiovascular abnormalities, either in general or specifically related to heart rate abnormalities.
The Spanish database for suspected adverse drug reactions Farmacovigiliancia Española, de Datos de Reacciones Adversas (Spanish Pharmacovigilance, Adverse Reaction Data, FEDRA)6 only contains 2 cases of heart rate abnormalities associated with rupatadine, although none are listed as TdP or QT prolongation. Therefore, this adverse reaction has been considered the first case of this rhythm disorder related to rupatadine entered into the Spanish Pharmacovigilance System.