Clinical decision-making on anticoagulation in elderly patients with atrial fibrillation (AF) requires clinicians to consider not only the incidence of embolic and bleeding events, but also the risk of death following these adverse events. We aimed to analyze the trade-off between embolic and bleeding events with respect to mortality in elderly patients with AF.
MethodsThe study cohort comprised all patients aged ≥ 75 years from a Spanish health area diagnosed with AF between 2014 and 2017 (n=9365). The risk of death was investigated using Cox proportional hazards models, including embolic and bleeding events as time-dependent binary indicators.
ResultsDuring a median follow-up of 4.0 years, both embolic and bleeding events were associated with a higher risk of death (adjusted HR, 2.39; 95%CI, 2.12-2.69; and adjusted HR, 1.79; 95%CI, 1.64-1.96, respectively). The relative risk of death was 33% higher following an embolism than following a bleeding event (rRR, 1.33; 95%CI, 1.15-1.55), although for transient ischemic attack the risk was lower than for bleeding (rRR, 0.79; 95%CI, 0.63-0.99). The risk of death associated with intracranial hemorrhage was similar to that of major embolisms (RR, 1.00; 95%CI, 0.75-1.29).
ConclusionsIn elderly AF patients, embolic events appeared to be associated with a higher risk of mortality than extracranial bleeding, except for transient ischemic attacks, which have a better prognosis. For ICH, the mortality risk was similar to that of major embolism.
Keywords
Oral anticoagulation (OAC) reduces the frequency of embolic events in patients with atrial fibrillation (AF). However, this benefit is counterbalanced by an increase in bleeding. Bleeding was historically considered an acceptable price to pay for OAC therapy; however, it has recently been shown to independently impact mortality.1
Advancing age affects multiple facets of the risk-benefit assessment of anticoagulation for AF patients. Consensus guidelines categorize all patients aged 75 years and older as being at high risk for ischemic stroke, thus warranting anticoagulation after a qualitative assessment of the risk of hemorrhage.2 However, decision-making regarding antithrombotic prophylaxis in these patients creates a dilemma for many physicians, as elderly patients are a heterogeneous group whose disease is complicated by the presence of functional and social factors that contribute to vulnerability and who experience multiple comorbidities and require polypharmacy.3 Despite observational studies and trials showing that the benefits of OAC extend to the oldest segments of the AF population,4 real-world data consistently show that the OAC prescription rate is inversely related to age.5 A better understanding of patients’ risk profiles is therefore needed to fill the gap between epidemiological evidence and clinical practice.
While the effects on the incidence of embolic and bleeding events dominate the decision to prescribe anticoagulation in patients with AF, the severity of such events should also be incorporated into clinical decision-making for individual patients and formal analyses, especially in complex patients such as the elderly. It is critical to understand the prognostic implications of embolic events, especially ischemic stroke, relative to bleeding outcomes in order to assist clinicians in selecting candidates for OAC treatment. However, the long-term impact of bleeding in AF patients has been less extensively investigated than the impact of embolic events.6 Similarly, its effect on mortality in terms of severity and the risk of death following an embolic event remains unclear. In this study, which is based on a large community registry of AF patients, we aimed to compare the relative impact of embolic and bleeding events on all-cause mortality.
METHODSStudy populationThe study cohort comprised all patients aged ≥ 75 years from the health area of Vigo (Galicia, Spain) diagnosed with AF between January 2014 and December 2017 (CardioCHUVI-AF_75 registry; ClinicalTrials.gov Identifier: NCT04364516). Electronic medical records were meticulously reviewed to confirm the diagnosis of AF and collect data on baseline clinical variables and therapeutic strategy. In all patients, the diagnosis of AF was confirmed only when it was based on an electrocardiogram. The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the local ethics committee (Autonomous Committee of Research Ethics from Galicia, code HAC-ACO-2018-01, registry 2018/258). Informed consent was not required for the present study.
OutcomesThis was a retrospective follow-up study. The primary outcome was all-cause mortality during follow-up. Follow-up was performed from the first time the patient was medically assessed for AF between January 2014 and December 2017, and lasted until June 2020. Deaths were adjudicated as either cardiovascular or noncardiovascular. Cardiovascular death includes death resulting from acute myocardial infarction, sudden cardiac death, death due to heart failure, death due to stroke, death due to cardiovascular procedures, death due to cardiovascular hemorrhage, and death due to other cardiovascular causes.7 We aimed to assess the association between mortality and a) any embolic event, and b) any bleeding event. Embolic events included ischemic stroke, transient ischemic attack (TIA), pulmonary embolism, and systemic embolism. Both pulmonary embolism and systemic embolism were classified as noncentral nervous system (non-CNS) embolisms. Major embolism included the combination of ischemic stroke and non-CNS embolism.7 Bleeding events were classified according to the statement of the International Society on Thrombosis and Haemostasis (ISTH) and were classified as major bleeding (MB) and clinically relevant non-MB.8,9 According to location, MB was further classified as intracranial hemorrhage (ICH) and non-ICH MB. More information about the definition of each embolic and bleeding event is detailed in the title “.
Statistical analysesBaseline variables were summarized by whether an embolism or bleeding event occurred during follow-up. Continuous data are presented as mean±standard deviation and were compared using unpaired t tests. Categorical data are presented as counts (proportions) and were compared using chi-square tests. Venn diagrams were used to illustrate the relationship between bleeding and embolic events.
The risk of all-cause mortality was investigated using Cox proportional hazards models. All the models described below included embolic and bleeding events as time-dependent binary indicators, as well as significant confounding variables as covariates. Demographic, anthropometric, clinical, echocardiographic, and laboratory variables, as well as medical therapy, were tested as potential confounding variables (). The best-fitting model was selected based on the Akaike information criterion. Hazard ratios (HRs) and P-values for the risk of all-cause mortality associated with bleeding and embolic events were obtained. The risk of recurrent events was modelled taking into account the first occurrence of such an event, whereas events of different severity (non-MB followed by ICH), were considered separate covariates. For patients who experienced a bleeding and an embolic event, both events were counted, and each was considered individually as a predictor of death (ie, the models included covariates for bleeding and embolic events). The relative hazard of embolic and bleeding events was estimated from the same models, and was expressed as the relative risk ratio (rRR).10
Additional analyses were performed to investigate the time-dependent nature of the risk of death associated with bleeding and embolic events as a function of the time elapsed since the event. For this purpose, we used the Royston-Parmar survival approach with flexible parametric survival models. The functional form of the relationship between the adjusted hazard of death and the time elapsed since bleeding or embolic events was adjusted using fractional polynomials, and the resulting curve was plotted along with the 95% confidence interval [95%CI] of the mean.
All tests were 2-sided, and statistical significance was set at P <.05. All analyses were performed using STATA software, version 15 (Stata Corp, College Station, United States).
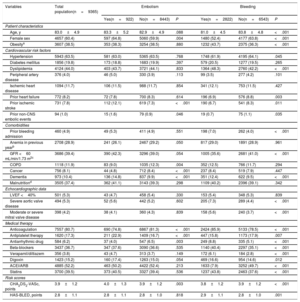
RESULTSWe studied 9365 patients aged ≥ 75 years with a confirmed diagnosis of AF. A total of 7557 patients were anticoagulated (80.7%). Of them, 6360 were anticoagulated with vitamin K antagonists (84.2%), 1070 with direct oral anticoagulants (14.1%) and 127 with heparin (1.7%). For those patients treated with vitamin K antagonists, the percentage of international normalized ratio in the therapeutic range (between 2 and 3) was 45.2%. For those patients treated with direct oral anticoagulants, 30.2% of patients received an inappropriate direct oral anticoagulants dose. During a median follow-up of 4.0 [interquartile range, 2.1-5.2] years, 2822 patients (30.1%) experienced bleeding events, and 922 patients (9.8%) embolic events. The baseline characteristics of patients with follow-up embolic and bleeding events are summarized in table 1.
Baseline demographic and clinical characteristics by occurrence of embolism and bleeding
| Variables | Total population(n=9365) | Embolism | Bleeding | ||||
|---|---|---|---|---|---|---|---|
| Yes(n=922) | No(n=8443) | P | Yes(n=2822) | No(n=6543) | P | ||
| Patient characteristics | |||||||
| Age, y | 83.0±4.9 | 83.3±5.2 | 82.9±4.9 | .088 | 81.0±4.5 | 83.8±4.8 | <.001 |
| Female sex | 4657 (60.4) | 597 (64.8) | 5060 (59.9) | .004 | 1480 (52.4) | 4177 (63.8) | <.001 |
| Obesitya | 3607 (38.5) | 353 (38.3) | 3254 (38.5) | .880 | 1232 (43.7) | 2375 (36.3) | <.001 |
| Cardiovascular risk factors | |||||||
| Hypertension | 5943 (63.5) | 581 (63.0) | 5365 (63.5) | .768 | 1748 (61.9) | 4195 (64.1) | .045 |
| Diabetes mellitus | 1856 (19.8) | 173 (18.8) | 1683 (19.9) | .397 | 579 (20.5) | 1277 (19.5) | .265 |
| Dyslipidemia | 4124 (44.0) | 403 (43.7) | 3721 (44.1) | .833 | 1364 (48.3) | 2760 (42.2) | <.001 |
| Peripheral artery disease | 376 (4.0) | 46 (5.0) | 330 (3.9) | .113 | 99 (3.5) | 277 (4.2) | .101 |
| Ischemic heart disease | 1094 (11.7) | 106 (11.5) | 988 (11.7) | .854 | 341 (12.1) | 753 (11.5) | .427 |
| Prior heart failure | 772 (8.2) | 72 (7.8) | 700 (8.3) | .614 | 196 (6.9) | 576 (8.8) | .003 |
| Prior ischemic stroke | 731 (7.8) | 112 (12.1) | 619 (7.3) | <.001 | 190 (6.7) | 541 (8.3) | .011 |
| Prior non-CNS embolic events | 94 (1.0) | 15 (1.6) | 79 (0.9) | .046 | 19 (0.7) | 75 (1.1) | .035 |
| Comorbidities | |||||||
| Prior bleeding admission | 460 (4.9) | 49 (5.3) | 411 (4.9) | .551 | 198 (7.0) | 262 (4.0) | <.001 |
| Anemia in previous yearb | 2708 (28.9) | 241 (26.1) | 2467 (29.2) | .050 | 817 (29.0) | 1891 (28.9) | .961 |
| GFR <60 mL/min/1.73 m2c | 3686 (39.4) | 390 (42.3) | 3296 (39.0) | .054 | 1005 (35.6) | 2681 (41.0) | <.001 |
| COPD | 1118 (11.9) | 83 (9.0) | 1035 (12.3) | .004 | 352 (12.5) | 766 (11.7) | .294 |
| Cancer | 756 (8.1) | 44 (4.8) | 712 (8.4) | <.001 | 237 (8.4) | 519 (7.9) | .447 |
| Dementia | 973 (10.4) | 136 (14.8) | 837 (9.9) | <.001 | 351 (12.4) | 622 (9.5) | <.001 |
| Malnutritiond | 3505 (37.4) | 362 (41.1) | 3143 (39.3) | .298 | 1109 (40.2) | 2396 (39.1) | .342 |
| Echocardiographic data | |||||||
| LVEF <40% | 501 (5.3) | 43 (4.7) | 458 (5.4) | .330 | 153 (5.4) | 348 (5.3) | .839 |
| Severe aortic valve disease | 494 (5.3) | 52 (5.6) | 442 (5.2) | .602 | 205 (7.3) | 289 (4.4) | <.001 |
| Moderate or severe mitral valve disease | 398 (4.2) | 38 (4.1) | 360 (4.3) | .839 | 158 (5.6) | 240 (3.7) | <.001 |
| Medical therapy | |||||||
| Anticoagulation | 7557 (80.7) | 690 (74.8) | 6867 (81.3) | <.001 | 2424 (85.9) | 5133 (78.5) | <.001 |
| Antiplatelet therapy | 1620 (17.3) | 211 (22.9) | 1409 (16.7) | <.001 | 447 (15.8) | 1173 (17.9) | .007 |
| Antiarrhythmic drug | 584 (6.2) | 37 (4.0) | 547 (6.5) | .003 | 249 (8.8) | 335 (5.1) | <.001 |
| Beta-blockers | 3437 (36.7) | 347 (37.6) | 3090 (36.6) | .535 | 1140 (40.4) | 2297 (35.1) | <.001 |
| Verapamil/diltiazem | 356 (3.8) | 43 (4.7) | 313 (3.7) | .149 | 172 (6.1) | 184 (2.8) | <.001 |
| Digoxin | 1423 (15.2) | 160 (17.4) | 1263 (15.0) | .054 | 469 (16.6) | 954 (14.6) | .012 |
| ACEI/ARB | 4885 (52.2) | 463 (50.2) | 4422 (52.4) | .213 | 1633 (7.9) | 3252 (49.7) | <.001 |
| Statins | 3700 (39.5) | 373 (40.5) | 3327 (39.4) | .536 | 1237 (43.8) | 2463 (37.6) | <.001 |
| Risk scores | |||||||
| CHA2DS2-VASc, points | 3.9±1.2 | 4.0±1.3 | 3.9±1.2 | .003 | 3.8±1.2 | 3.9±1.2 | <.001 |
| HAS-BLED, points | 2.8±1.1 | 2.8±1.1 | 2.8±1.0 | .818 | 2.9±1.1 | 2.8±1.0 | .001 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes, stroke [doubled], vascular disease, age 65 to 74 years, and sex category [female]; CNS, central nervous system; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; HAS-BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly; LVEF, left ventricular ejection fraction.
Data are expressed as No. (%) for discrete variables and mean±standard deviation for continuous variables.
According to the World Health Organization, anemia was defined as hemoglobin levels <12.0g/dL in women and <13.0g/dL in men. We did not include anemia following traumatic injuries or surgical treatments.
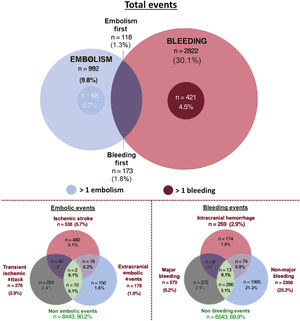
Most of the bleeding events were non-MB (n=2368; 83.9% of total bleeding events), whereas most of the embolisms were ischemic strokes (n=538; 58.3% of total embolic events) (more information in ). ICH was recorded in 269 patients (2.9%). More than 1 bleeding event occurred in 4.5% of patients (n=421) during follow-up, whereas multiple embolic events occurred in 0.7% (n=68) (figure 1). The percentage of patients experiencing both bleeding and ischemic stroke during follow-up was 3.1% (n=291), with similar percentages experiencing a bleeding event first (1.8%) or an embolism first (1.3%).
Distribution of bleeding events and embolisms in the study population. The Venn diagram shows patients experiencing bleeding (in red) and embolisms (in blue). Smaller circles represent patients experiencing more than 1 event during follow-up. The intersection represents patients experiencing both embolisms and bleeding during follow-up.
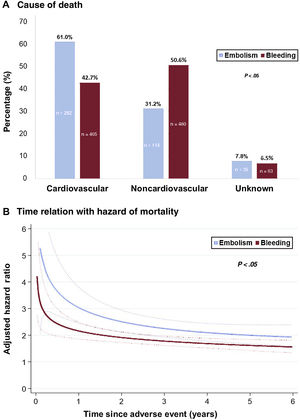
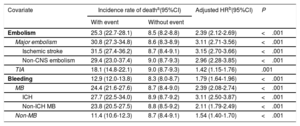
A total of 3146 patients (33.6%) died. Both bleeding and embolic events were associated with a higher risk of mortality (table 2). Patients with any type of bleeding (MB and clinically relevant non-MB) had a 79% higher risk of death than those without bleeding events (adjusted HR, 1.79; 95%CI, 1.64-1.96; P <.001). Although the highest risk of death was after an ICH (adjusted HR, 3.11; 95%CI, 2.50-3.87; P <.001), clinically relevant non-MB was also associated with a significant increase in the risk of mortality (adjusted HR, 1.54; 95%CI, 1.40-1.70; P <.001). Patients with an embolic event had a 2-fold increase in the hazard of death (adjusted HR, 2.39; 95%CI, 2.12-2.69; P <.001). The highest risk of mortality following an embolism was for ischemic stroke (adjusted HR, 3.11; 95%CI, 2.50-3.87; P <.001), and the lowest risk for TIA (adjusted HR, 1.42; 95%CI, 1.15-1.76; P <.001). After an embolism, most of the deaths were cardiovascular (61.0%), whereas after a bleeding event, most of deaths were noncardiovascular (50.6%) (figure 2A). For both bleeding and embolism, the risk of death was higher early after the event; it dissipated after the first year, but it still maintained a significant prognostic impact for several years thereafter (figure 2B).
Association between risk of death and bleeding and embolic events
| Covariate | Incidence rate of deatha(95%CI) | Adjusted HRb(95%CI) | P | |
|---|---|---|---|---|
| With event | Without event | |||
| Embolism | 25.3 (22.7-28.1) | 8.5 (8.2-8.8) | 2.39 (2.12-2.69) | <.001 |
| Major embolism | 30.8 (27.3-34.8) | 8.6 (8.3-8.9) | 3.11 (2.71-3.56) | <.001 |
| Ischemic stroke | 31.5 (27.4-36.2) | 8.7 (8.4-9.1) | 3.15 (2.70-3.66) | <.001 |
| Non-CNS embolism | 29.4 (23.0-37.4) | 9.0 (8.7-9.3) | 2.96 (2.28-3.85) | <.001 |
| TIA | 18.1 (14.8-22.1) | 9.0 (8.7-9.3) | 1.42 (1.15-1.76) | .001 |
| Bleeding | 12.9 (12.0-13.8) | 8.3 (8.0-8.7) | 1.79 (1.64-1.96) | <.001 |
| MB | 24.4 (21.6-27.6) | 8.7 (8.4-9.0) | 2.39 (2.08-2.74) | <.001 |
| ICH | 27.7 (22.5-34.0) | 8.9 (8.7-9.2) | 3.11 (2.50-3.87) | <.001 |
| Non-ICH MB | 23.8 (20.5-27.5) | 8.8 (8.5-9.2) | 2.11 (1.79-2.49) | <.001 |
| Non-MB | 11.4 (10.6-12.3) | 8.7 (8.4-9.1) | 1.54 (1.40-1.70) | <.001 |
95%CI, 95% confidence interval; CNS, central nervous system; HR, hazard ratio; ICH, intracranial hemorrhage; MB, major bleeding; TIA, transient ischemic attack.
Adjustments were finally applied for the significant influential characteristics of age, female sex, obesity, cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidemia), peripheral artery disease, ischemic heart disease, prior heart failure, prior embolic events (ischemic stroke and extracranial embolism), comorbidities (anemia in the last year, glomerular filtrate rate according to Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] equation <60mL/min/1.73 m2, chronic obstructive pulmonary disease, history of cancer, dementia, risk of malnutrition [Controlling Nutritional Status—CONUT—score ≥ 3]), echocardiographic data (left ventricular ejection fraction <40%, severe aortic and mitral valve disease), medical therapies (anticoagulation, antiplatelet therapy, antiarrhythmic drugs, beta-blockers, verapamil/diltiazem, digoxin, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and statins), and risk scores (CHA2DS2-VASc and HAS-BLED).
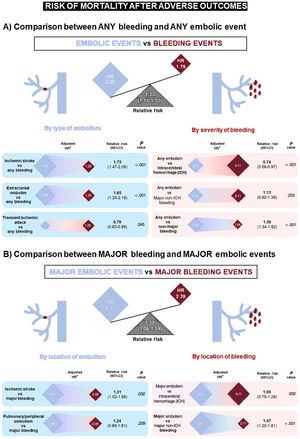
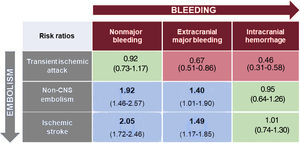
The relative impact on mortality of an embolic vs a bleeding event is displayed graphically in figure 3A. The relative hazard of death was 33% higher in patients experiencing an embolism vs a bleeding event (2.39 vs 1.79; rRR, 1.33; 95%CI, 1.15-1.55; P <.001). The increased mortality risk observed after an embolic event vs a bleeding event has been also maintained when the analyses were limited to patients with major adverse events (figure 3B). When different embolic vs hemorrhagic events were compared one by one, the risk of mortality after an adverse event was always higher for embolic than hemorrhagic episodes, except for ICH (higher risk than TIA, similar risk to major embolism) and TIA (lower risk than MB, similar risk to non-MB) (figure 4). The impact of anticoagulation therapy at the time of bleeding or embolic event in the hazard of mortality was also assessed (). The relative risk of mortality for an embolism compared with a bleeding event was similar between patients on and off anticoagulation [rRR, 1.34; 95%CI, 1.13-1.60; P <.001] and 1.23 [95%CI, 0.97-1.57; P=.084], respectively; P value for the interaction .686), without differences between patients treated with vitamin K antagonists and those treated with direct oral anticoagulants ().
Differential impact of embolic vs bleeding events on mortality. A: blue rhombuses represent the magnitude (adjusted hazard ratio [HR]) of the impact on mortality of different types of embolic events, whereas red rhombuses represent that of bleeding of different degrees of severity. We reported the estimate of the relative risk (ratio of the HR) for each comparison. B: comparison is focused only on major events. Major bleeding (MB) includes intracranial hemorrhage (ICH) and non-ICH MB. Major embolism includes ischemic stroke, pulmonary embolism, and peripheral arterial embolism. 95%CI, 95% confidence interval. *The estimates of the impact of events on mortality are derived from a multivariate model (see table 1). ICH, intracranial hemorrhage.
Estimates of the relative risk (ratio of the hazard ratios) of mortality for the comparison between different bleeding and embolic events according to their severity. The estimates of the impact of events on mortality are derived from a multivariate model in which each event was considered individually as a time-dependent binary indicator of death, in addition to the remaining covariates (for multivariate adjustment, see table 1). CNS, central nervous system.
To our knowledge, this is the first real-world observational study to compare the prognostic impact on mortality of embolism and bleeding in a cohort of elderly AF patients. Our principal findings are as follows: a) both bleeding and embolic events are associated with an increased risk of death; b) embolic events had higher prognostic value for death than bleeding events, both for total and major episodes, although this value depended on the type of embolism and bleeding; c) the mortality rate for TIA was lower than that of MB, and the mortality rate for ICH was similar to that of major embolism (ischemic stroke or non-CNS embolism); d) the main cause of death following an adverse event was different for embolisms than for bleeding (noncardiovascular); e) the time pattern of the associated risk of death was similar between bleeding and embolic events, with the risk being higher early after the event and diminished after the first year, although with significant prognostic impact for several years thereafter.
In our study, embolic events nearly triple the risk of death in patients with AF, with most patients dying within the first year after the event. Although all embolic events were associated with a worse long-term prognosis, the highest rate of mortality following an adverse event was for ischemic stroke. Numerous studies have documented a high risk of death for AF-related ischemic stroke, even in the long-term.6,11 In the ATRIA AF cohort, 40% of patients sustaining an ischemic stroke had died within 1 year after the stroke.6 In our study, with a more contemporary cohort of patients, we report an incidence rate of death following ischemic stroke of 31.5 per 100 patients per year, and 18.1 per 100 patients per year after a TIA. The association between TIA and mortality has been documented elsewhere, with a decrease in short- and long-term survival of 5% in the first year and 20% within 10 years.12,13 In contrast to the well-characterized risk and sequelae of cerebral embolism, much less is known about the outcomes associated with non-CNS embolic events in AF. Using retrospectively pooled data from 4 published randomized trials, Bekwelem et al.14 showed that 30-day mortality was similar in patients with systemic embolic events or stroke. We confirmed these results from clinical trials in a real-world population, and our findings held during the long-term follow-up. In our study, the adjusted HR for mortality of both ischemic stroke and non-CNS embolism was similar. Therefore, and even though observational studies and randomized trials have focused almost entirely on stroke prevention, both ischemic stroke and non-CNS embolisms (systemic embolism and pulmonary embolism) should be considered equally as major embolic events.
In contrast to the emphasis on prognosis associated with embolic episodes, detailed analyses of long-term survival after bleeding in the setting of AF have rarely been reported. Prior studies have demonstrated that it is ICH that overwhelmingly determines poor outcomes in patients receiving OAC,15 and, as a result, the risk of non-ICH MB should have a relatively small effect on decisions about OAC therapy in AF. Although extracranial hemorrhages certainly result in hospitalizations, invasive procedures, and discontinuation of antithrombotic therapy, the longer-term functional impact of such hemorrhages is generally considered much lower than that of ICH or ischemic stroke. However, recent data from the ARISTOTLE trial showed that both non-ICH MB and clinically relevant non-MB are associated with a substantially increased risk of death at 30 days.16,17 We confirmed that not only ICH but also MB and non-MB were independently associated with a significantly higher risk of death. Interestingly, our findings clearly show that the negative effect of bleeding on survival persists far beyond the initial event, thus demonstrating that 30-day case fatality rates are a conservative estimate of the effect of bleeding on survival.
Beyond looking at the independent prognosis of embolisms and bleeding, our study balances for the first time the long-term risk of death associated with embolisms and bleeding. This approach is particularly interesting when assessing the risk of death and is crucial when making decisions on anticoagulation therapy, especially in complex populations at high risk for both embolic and bleeding events (eg, elderly patients). We show that the weight of mortality from embolic events (except for TIA) is greater than that of bleeding events (except for ICH). This is consistent with subjective perception. Devereaux et al.18 revealed that both patients and physicians are willing to accept a far higher risk of non-ICH bleeding in return for an associated reduction in the risk of stroke.
Choosing anticoagulation therapy for patients with AF depends to a large extent on balancing the reduction in the rate of embolic events expected after OAC with the increased rate of bleeding. Net clinical benefit has become a popular endpoint to account for the effect of both efficacy and bleeding. However, there is an intrinsic risk of misinterpretation when components are heterogeneous in terms of importance, number of events, or magnitude of treatment effect. In this scenario, one can imagine that if the directions of non-MB and ischemic stroke are different, but non-MB occurs more frequently, the net clinical benefit will be pushed toward the treatment with fewest events, irrespective of their clinical significance. Therefore, in studies that aim to rank or weight events according to their clinical significance, minimizing imbalances resulting from differences in the direction and magnitude of the individual components of the endpoint is of great importance.10 However, the evidence on how various bleeding types should be weighted in combined endpoints against different types of embolism has been limited to date. Hence, our findings could prove useful in that they indicate a more objective way of weighting bleeding and embolic events. Data on the mortality expected from AF-associated embolisms compared with bleeding events are vital when assessing the results of clinical trials and attempting to improve how clinicians and patients select antithrombotic therapy for AF. Physicians should inform patients about the risks of death associated with embolism and bleeding to facilitate shared decision-making about anticoagulation, especially in complex patients, such as the elderly. Our findings can modify thresholds—for both physicians and patients—with respect to the risk of excess bleeding deemed acceptable with antithrombotic treatments and the reduction in the risk of embolism thought necessary to justify treatment with OAC.
Strengths and weaknesses of the studyOur study reflects the experience of a large, community-based cohort of real-world elderly patients with AF followed up for more than 4 years. Ours was therefore an unselected patient population that likely has a higher risk of mortality and bleeding than unselected patients in clinical practice. The events in our study were validated by a review of the clinical histories, which reduces the potential risk of misclassification associated with reliance on administrative codes alone. Our analysis was based on a widely accepted and reproducible definition of bleeding, which takes into account the degree of severity of bleeding in order to compare the relative risks of death. We consider not only the severity of bleeding events but also the severity and type of embolic events. Interestingly, and unlike most studies on the prognostic impact of bleeding, we did not limit outcomes to 30 days. We analyzed the temporal association with mortality for a median follow-up of 4 years. Finally, we reported relevant data about how mortality varies according to exposure to OAC. Taken together, these factors confer high clinical value on our findings.
Our study also has a series of limitations. First, it is subject to the limitations that are inherent to its observational, retrospective nature. Second, the interplay between bleeding, embolism, and mortality is highly complex, and we were unable to account for all possible factors involved in their causal relationship. In this regard, we have no data about how a hemorrhagic transformation of an ischemic stroke accounts for the excessive mortality risk of major embolism compared with extracranial MB. Third, we had no data on nuisance bleeding, although this is unlikely to change our results, as nuisance bleeding does not seem to be associated with a worse prognosis in terms of mortality.19 Fourth, we have no data about therapeutic changes in anticoagulation after the embolic or bleeding event. Finally, we focused on mortality as our primary endpoint; we did not explore other interesting endpoints, such as the impact of bleeding and embolic events on quality of life.
CONCLUSIONSIn elderly patients with AF, both embolic and bleeding events significantly impacted mortality within a similar time framework. Embolic events appeared to be associated with a higher risk of mortality than extracranial bleeding, except for TIAs, which have a better prognosis. For ICH, the subsequent risk of death was similar to that of major embolism. These findings may help clinicians to interpret the risk-benefit profile of anticoagulant drugs.
FUNDINGDaiichi Sankyo, Bayer, Boehringer Ingelheim, and Pfizer, have provided unconditional financial support for this study.
AUTHORS’ CONTRIBUTIONSS. Raposeiras-Roubín: study design, data analysis, manuscript writing, and article submission. E. Abu-Assi: study design, data analysis and critical review of the article. M. Cespón Fernández: data collection and critical review of the article. S. Blanco Prieto: data collection and critical review of the article. C. Barreiro Pardal: data collection and critical review of the article. P. Domínguez-Erquicia: data collection and critical review of the article. M. Melendo Viu: data collection and critical review of the article. C. Bonanad Lozano: data analysis and critical review of the article. X. Rosselló: data analysis and critical review of the article. B. Ibáñez: study design and critical review of the article. A. Íñiguez Romo: study design and critical review of the article.
CONFLICTS OF INTERESTE. Abu-Assi is associate editor of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed.
- -
Both embolic events and bleeding events are associated with a worse prognosis in patients with atrial fibrillation. The impact on mortality has been confirmed both in the short- and long-term, especially for embolic events. However, which events have more impact on the mortality: embolisms or hemorrhages?
- -
This is the first study to compare long-term mortality associated with embolic vs bleeding events in a population of elderly patients with atrial fibrillation. A comparative analysis of the impact on mortality was carried out according to the type of embolism and the type of bleeding. In general, embolic events appeared to be associated with a higher risk of mortality than bleeding events. This should make us reflect on the need to consider anticoagulation in elderly patients, regardless of their age.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.02.003



![Differential impact of embolic vs bleeding events on mortality. A: blue rhombuses represent the magnitude (adjusted hazard ratio [HR]) of the impact on mortality of different types of embolic events, whereas red rhombuses represent that of bleeding of different degrees of severity. We reported the estimate of the relative risk (ratio of the HR) for each comparison. B: comparison is focused only on major events. Major bleeding (MB) includes intracranial hemorrhage (ICH) and non-ICH MB. Major embolism includes ischemic stroke, pulmonary embolism, and peripheral arterial embolism. 95%CI, 95% confidence interval. *The estimates of the impact of events on mortality are derived from a multivariate model (see table 1). ICH, intracranial hemorrhage. Differential impact of embolic vs bleeding events on mortality. A: blue rhombuses represent the magnitude (adjusted hazard ratio [HR]) of the impact on mortality of different types of embolic events, whereas red rhombuses represent that of bleeding of different degrees of severity. We reported the estimate of the relative risk (ratio of the HR) for each comparison. B: comparison is focused only on major events. Major bleeding (MB) includes intracranial hemorrhage (ICH) and non-ICH MB. Major embolism includes ischemic stroke, pulmonary embolism, and peripheral arterial embolism. 95%CI, 95% confidence interval. *The estimates of the impact of events on mortality are derived from a multivariate model (see table 1). ICH, intracranial hemorrhage.](https://static.elsevier.es/multimedia/18855857/0000007500000004/v1_202204020646/S1885585721000670/v1_202204020646/en/main.assets/thumbnail/gr3.jpeg?xkr=eyJpdiI6IjRKcWJTQW9SV0FzTkkwQXVubzhMbmc9PSIsInZhbHVlIjoiOE5xcUtxa1V1OVQxcnhPTWRLQkR5L1pQbUlFSW9oT1FndWp6SHVuQUh0MD0iLCJtYWMiOiJhZDA5M2Q2YjE4NmQ5MWI4MTJmZDM1YmYwZDdkZmE1OWJmZDQwZTkzMDkxZmQ3ZjkyOWM2Mzg1OGEyZTYyZmY5IiwidGFnIjoiIn0=)
