Transcatheter mitral valve repair (TMVR) with MitraClip is a therapeutic option for high surgical risk patients with severe mitral regurgitation (MR). The main objective of this study was to analyze differences in outcomes in patients with severe MR according to the cause of MR.
MethodsObservational, multicenter, and prospective study with consecutive patient inclusion. The primary endpoint was the combination of all-cause mortality and new readmissions due to heart failure after 1 year. We compared clinical and procedural characteristics and the event rate for each MR group. We performed a multivariate analysis to identify predictive variables for the primary endpoint.
ResultsA total of 558 patients were included: 364 (65.2%) with functional etiology, 111 (19.9%) degenerative and 83 (14.9%) mixed. The mean age was 72.8±11.1 years and 70.3% of the sample were men. There were 95 (17%) events in the overall sample. No significant differences were found in the 3 groups in the number of primary outcome events: 11 (11.3%) in degenerative MR, 71 (21.3%) in functional MR, and 13 (18.1%) in mixed MR (P=.101). Independent predictors were functional class (P=.029), previous surgical revascularization (P=.031), EuroSCORE II (P=.003), diabetes mellitus (P=.037), and left ventricular ejection fraction (P=.015).
ConclusionsThis study confirms the safety and efficacy of TMVR with MitraClip irrespective of MR etiology in real-life data and shows the main factors related to prognosis during the first year of follow up.
Keywords
Mitral regurgitation (MR) is a valve disease with increasing prevalence. Severe MR is associated with progressive left ventricular dilatation and the development of heart failure (HF). Untreated symptomatic patients have annual mortality rates exceeding 5%.1,2 MR interventions vary according to the pathophysiological mechanism: in primary or organic MR, some of the components of the mitral apparatus (leaflets, chordae, or papillary muscles) are affected and valve repair/replacement is recommended when there are symptoms, ventricular dilatation, pulmonary hypertension, or atrial fibrillation.3,4 In secondary MR, the components of the mitral apparatus are intact but leaflet coaptation fails due to ventricular or ring dilatation. These patients usually have ventricular dysfunction, and most receive medical therapy. Surgical treatment is only considered when concomitant coronary revascularization is required.3–5
The emergence of transcatheter mitral valve repair (TMVR) techniques has bolstered the therapeutic arsenal for the treatment of severe MR. The MitraClip system (Abbott; Menlo Park, California, United States) is an increasingly widespread therapeutic option for severe MR patients who are at high surgical risk or are inoperable.4,5 The management of patients with severe MR of degenerative origin with TMVR with the MitraClip is safe and effective.6 The approach achieves good clinical outcomes, with a reduction in MR severity that is slightly lower in the long-term vs that achieved by surgical treatment, but shows greater safety and improved functional class and quality of life.7,8
Although the initial MitraClip evidence was obtained in the field of degenerative MR (DMR), most patients treated in international registries had functional MR (FMR). In this population, an improved functional class was observed in more than 75% of patients.9–12 This led to the comparison of TMVR plus optimal medical therapy vs optimal medical therapy alone in 2 randomized studies. While in one of them (COAPT), the TMVR plus optimal medical therapy group showed significantly reduced mortality and readmissions for HF, the other (MITRA-FR) found no significant differences.13,14
Thus, the main objective of this study was to analyze the differences in 1-year all-cause mortality and hospital readmissions for HF in patients with degenerative, functional, and mixed MR managed with TMVR with the MitraClip system.
METHODSStudy design and populationIn this observational and multicenter study, data were obtained from the Spanish MitraClip registry. This registry is endorsed by the Cardiac Catheterization and Interventional Cardiology (SHCI) Section of the Spanish Society of Cardiology and has prospectively included consecutive patients treated with MitraClip since June 1, 2012. The inclusion of patients treated with the MitraClip in the Spanish registry is open to all members of the SHCI who perform the technique. This study analyzed the data of the patients included until July 1, 2018, from 16 Spanish hospitals. The indication for TMVR with MitraClip was established as the best therapeutic option after evaluation of each patient by the multidisciplinary team (Heart Team) of each center.
For the purpose of the study, 3 groups were defined according to MR cause3,4: primary MR or DMR, which entails a direct and structural involvement of some of the components of the mitral apparatus; secondary MR or FMR, which shows structurally normal leaflets and chordae but an imbalance between the closing and tethering forces in the valve, secondary to alterations in left ventricular geometry; and finally, mixed MR (MMR), whose etiological mechanism is mainly functional, but there is leaflet degeneration that could have implications for percutaneous repair.
A specialized centralized database was designed for the prospective and consecutive inclusion of all of the patients’ demographic, echocardiographic, procedural, and follow-up variables.
All included patients signed an informed consent form.
Description of the procedure and deviceThe MitraClip device is a percutaneous treatment system for MR that is based on the surgical technique proposed by Alfieri.6,7 The system consists of a cobalt-chromium clip with 2 arms and 2 grippers that are used to trap both mitral leaflets to create a double orifice valve. The device is transseptally introduced and, under echocardiographic and fluoroscopic visualization, its delivery system is advanced until it is placed at the site of maximum regurgitation. The use of real-time 3-dimensional transesophageal echocardiography is essential to guide the procedure and also makes it possible to attempt the treatment of morphologically complex valves. Once transesophageal echocardiography has confirmed that both leaflets have been introduced into the arms of the system, the device can be closed and the result of the MR reduction can be checked by transesophageal echocardiography. If the result is acceptable, the clip can be released; if not, it can be reopened and relocated for another attempt. The implantation is performed in the catheterization laboratory under general anesthesia to obtain an adequate image of the mitral valve and properly capture the leaflets in the device.6,7
Definition of the variablesProcedural success was defined as the correct implantation of at least 1 clip and MR reduction to a grade less than or equal to moderate (2+). Procedural time was defined as the duration from anesthetic induction to the end of the procedure. Device implantation time was calculated from the insertion of the release system until its removal. Procedure-related bleeding and its severity were defined according to the criteria of the Bleeding Academic Research Consortium (BARC).15 Functional class was defined according to the classification of the New York Heart Association (NYHA).
Percutaneous mitral repair was defined as urgent when it was performed during patient admission for acute HF and clinical instability, either due to recurrent acute lung edema episodes requiring intravenous medication or due to the development of cardiogenic shock.
MR severity, both at diagnosis and in the evaluation after the procedure and during follow-up, was determined according to the recommendations of the European Society of Cardiology.4,16
Study endpointsThe primary study endpoint was a composite of all-cause mortality and readmission for HF during the first year of follow-up.
The secondary objectives were post-TMVR analysis of functional class and MR severity according to its cause.
Statistical analysisIn the descriptive analysis, absolute (No.) and relative (%) frequencies were calculated for qualitative variables. For quantitative variables, the normality of the variables was assessed with the Kolmogorov-Smirnov goodness-of-fit test; variables are expressed as mean ± standard deviation if normally distributed and as median [interquartile range] if not. To address the study endpoints, analyses were performed to compare the 3 MR types (DMR, FMR, and MMR). The differences among these groups in the qualitative variables were calculated as a percentage difference with the Pearson chi-square test; if 20% or more of cells had expected frequencies < 5, likelihood ratio correction was performed. Comparisons among the 3 groups for continuous quantitative variables were analyzed by one-way ANOVA, with post hoc analysis of multiple Bonferroni comparisons or with the Kruskal-Wallis test according to the distribution of the variable.
The nonparametric Kaplan-Meier survival estimator was used to determine the time to all-cause mortality during 12 months of follow-up, as well as readmissions for HF and the composite of mortality and readmissions. In conjunction with the log-rank test, the median survival and the survival curves were used to compare the event-free survival rates among the groups according to MR cause.
Finally, independent predictors of the primary study endpoint were analyzed by Cox proportional hazards regression; hazard ratios (HRs) with 95% confidence intervals (95%CIs) were calculated for all patients, with adjustment for age, sex, type of MR, and the variables with P <.1 in the univariate analysis, using a stepwise forward model. A 95% level of significance was applied in the statistical tests (P <.05).
For data analysis, the SPSS version 23.0 statistical package was used (IBM Corp.; Armonk, New York, United States).
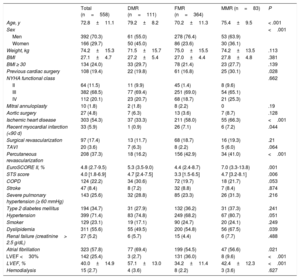
RESULTSBaseline characteristics of the populationIn total, 558 patients were included: 364 had FMR (65.2%), 111 had DMR (19.9%), and 83 had MMR (14.9%). Baseline characteristics are shown in table 1. The mean age was 72.8±11.1 years and men comprised 70.3%. Compared with patients with FMR, patients with DMR were older, were more likely to have had prior cardiac surgery, and had higher surgical risk. Among patients with FMR and MMR, there were higher proportions of men, previous ischemic heart disease, percutaneous revascularization, and previous surgery and worse ejection fraction.
Baseline characteristics of the sample
| Total (n=558) | DMR (n=111) | FMR (n=364) | MMR (n=83) | P | |
|---|---|---|---|---|---|
| Age, y | 72.8±11.1 | 79.2±8.2 | 70.2±11.3 | 75.4±9.5 | < .001 |
| Sex | <.001 | ||||
| Men | 392 (70.3) | 61 (55.0) | 278 (76.4) | 53 (63.9) | |
| Women | 166 (29.7) | 50 (45.0) | 86 (23.6) | 30 (36.1) | |
| Weight, kg | 74.2±15.3 | 71.5±15.7 | 75.0±15.5 | 74.2±13.5 | .113 |
| BMI | 27.1±4.7 | 27.2±5.4 | 27.0±4.4 | 27.8±4.8 | .381 |
| BMI ≥ 30 | 134 (24.0) | 33 (29.7) | 78 (21.4) | 23 (27.7) | .139 |
| Previous cardiac surgery | 108 (19.4) | 22 (19.8) | 61 (16.8) | 25 (30.1) | .028 |
| NYHA functional class | .662 | ||||
| II | 64 (11.5) | 11 (9.9) | 45 (1.4) | 8 (9.6) | |
| III | 382 (68.5) | 77 (69.4) | 251 (69.0) | 54 (65.1) | |
| IV | 112 (20.1) | 23 (20.7) | 68 (18.7) | 21 (25.3) | |
| Mitral annuloplasty | 10 (1.8) | 2 (1.8) | 8 (2.2) | 0 | .19 |
| Aortic surgery | 27 (4.8) | 7 (6.3) | 13 (3.6) | 7 (8.7) | .128 |
| Ischemic heart disease | 303 (54.3) | 37 (33.3) | 211 (58.0) | 55 (66.3) | <.001 |
| Recent myocardial infarction (<90 d) | 33 (5.9) | 1 (0.9) | 26 (7.1) | 6 (7.2) | .044 |
| Surgical revascularization | 97 (17.4) | 13 (11.7) | 68 (18.7) | 16 (19.3) | .21 |
| TAVI | 20 (3.6) | 7 (6.3) | 8 (2.2) | 5 (6.0) | .064 |
| Percutaneous revascularization | 208 (37.3) | 18 (16.2) | 156 (42.9) | 34 (41.0) | <.001 |
| EuroSCORE II, % | 4.8 (2.7-9.5) | 5.3 (3.5-9.0) | 4.4 (2.4-8.7) | 7.0 (3.3-13.8) | .001 |
| STS score | 4.0 [1.8-6.9] | 4.7 [2.4-7.5] | 3.3 [1.5-6.5] | 4.7 [3.2-8.1] | .006 |
| COPD | 124 (22.2) | 34 (30.6) | 72 (19.7) | 18 (21.7) | .053 |
| Stroke | 47 (8.4) | 8 (7.2) | 32 (8.8) | 7 (8.4) | .874 |
| Severe pulmonary hypertension (≥ 60 mmHg) | 143 (25.6) | 32 (28.8) | 85 (23.3) | 26 (31.3) | .216 |
| Type 2 diabetes mellitus | 194 (34.7) | 31 (27.9) | 132 (36.2) | 31 (37.3) | .241 |
| Hypertension | 399 (71.4) | 83 (74.8) | 249 (68.2) | 67 (80.7) | .051 |
| Smoker | 129 (23.1) | 19 (17.1) | 90 (24.7) | 20 (24.1) | .249 |
| Dyslipidemia | 311 (55.6) | 55 (49.5) | 200 (54.8) | 56 (67.5) | .039 |
| Renal failure (creatinine> 2.5 g/dL) | 27 (5.2) | 6 (5.7) | 15 (4.4) | 6 (7.7) | .488 |
| Atrial fibrillation | 323 (57.8) | 77 (69.4) | 199 (54.5) | 47 (56.6) | .021 |
| LVEF <30% | 142 (25.4) | 3 (2.7) | 131 (36.0) | 8 (9.6) | <.001 |
| LVEF, % | 40.0±14.9 | 57.1±13.0 | 34.2±11.4 | 42.4±12.3 | <.001 |
| Hemodialysis | 15 (2.7) | 4 (3.6) | 8 (2.2) | 3 (3.6) | .627 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; DMR, degenerative mitral regurgitation; FMR, functional mitral regurgitation; LVEF, left ventricular ejection fraction; MMR, mixed mitral regurgitation; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
Values represent No. (%), mean±standard deviation, mean (range), or median [interquartile range].
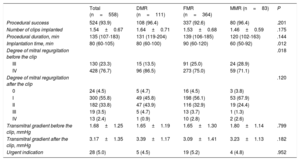
Procedural characteristics are shown in table 2. The procedure was successful in 93% of patients, without significant differences according to MR cause. There were also no significant differences in procedure duration, although the procedures were longer in the FMR group, or in the number of clips implanted. In 5% of the patients, the indication for TMVR was urgent, without significant differences according to type of MR.
Procedural characteristics
| Total (n=558) | DMR (n=111) | FMR (n=364) | MMR (n=83) | P | |
|---|---|---|---|---|---|
| Procedural success | 524 (93.9) | 108 (96.4) | 337 (92.6) | 80 (96.4) | .201 |
| Number of clips implanted | 1.54±0.67 | 1.64±0.71 | 1.53±0.68 | 1.46±0.59 | .175 |
| Procedural duration, min | 135 (107-183) | 131 (119-204) | 139 (106-185) | 120 (102-163) | .144 |
| Implantation time, min | 80 (60-105) | 80 (60-100) | 90 (60-120) | 60 (50-92) | .012 |
| Degree of mitral regurgitation before the clip | .018 | ||||
| III | 130 (23.3) | 15 (13.5) | 91 (25.0) | 24 (28.9) | |
| IV | 428 (76.7) | 96 (86.5) | 273 (75.0) | 59 (71.1) | |
| Degree of mitral regurgitation after the clip | .120 | ||||
| 0 | 24 (4.5) | 5 (4.7) | 16 (4.5) | 3 (3.8) | |
| I | 300 (55.8) | 49 (45.8) | 198 (56.1) | 53 (67.9) | |
| II | 182 (33.8) | 47 (43.9) | 116 (32.9) | 19 (24.4) | |
| III | 19 (3.5) | 5 (4.7) | 13 (3.7) | 1 (1.3) | |
| IV | 13 (2.4) | 1 (0.9) | 10 (2.8) | 2 (2.6) | |
| Transmitral gradient before the clip, mmHg | 1.68±1.25 | 1.65±1.19 | 1.65±1.30 | 1.80±1.14 | .799 |
| Transmitral gradient after the clip, mmHg | 3.17±1.35 | 3.39±1.17 | 3.09±1.41 | 3.23±1.13 | .182 |
| Urgent indication | 28 (5.0) | 5 (4.5) | 19 (5.2) | 4 (4.8) | .952 |
DMR, degenerative mitral regurgitation; FMR, functional mitral regurgitation; MMR, mixed mitral regurgitation;
Values represent No. (%) or mean±standard deviation.
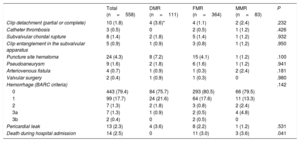
Procedural and in-hospital complications are shown in table 3. Procedure-related pericardial effusion was observed in 13 patients, 4 with DMR, 8 with FMR, and 1 with MMR. Of these, 4 patients required pericardiocentesis (2 with DMR and 2 with FMR). Only 1 patient in the FMR group required surgical treatment for pericardial effusion. There was clinically significant bleeding (BARC 3a or 3b) in 3.1% (9 patients). In-hospital mortality was 2.5% (14 patients) and was significantly higher in the FMR and MMR groups than in the DMR group (3% and 3.6% vs 0%; P=.041).
Intraprocedural and in-hospital complications
| Total (n=558) | DMR (n=111) | FMR (n=364) | MMR (n=83) | P | |
|---|---|---|---|---|---|
| Clip detachment (partial or complete) | 10 (1.8) | 4 (3.6)* | 4 (1.1) | 2 (2.4) | .232 |
| Catheter thrombosis | 3 (0.5) | 0 | 2 (0.5) | 1 (1.2) | .426 |
| Subvalvular chordal rupture | 8 (1.4) | 2 (1.8) | 5 (1.4) | 1 (1.2) | .932 |
| Clip entanglement in the subvalvular apparatus | 5 (0.9) | 1 (0.9) | 3 (0.8) | 1 (1.2) | .950 |
| Puncture site hematoma | 24 (4.3) | 8 (7.2) | 15 (4.1) | 1 (1.2) | .100 |
| Pseudoaneurysm | 9 (1.6) | 2 (1.8) | 6 (1.6) | 1 (1.2) | .941 |
| Arteriovenous fistula | 4 (0.7) | 1 (0.9) | 1 (0.3) | 2 (2.4) | .181 |
| Valvular surgery | 2 (0.4) | 1 (0.9) | 1 (0.3) | 0 | .980 |
| Hemorrhage (BARC criteria) | .142 | ||||
| 0 | 443 (79.4) | 84 (75.7) | 293 (80.5) | 66 (79.5) | |
| 1 | 99 (17.7) | 24 (21.6) | 64 (17.6) | 11 (13.3) | |
| 2 | 7 (1.3) | 2 (1.8) | 3 (0.8) | 2 (2.4) | |
| 3a | 7 (1.3) | 1 (0.9) | 2 (0.5) | 4 (4.8) | |
| 3b | 2 (0.4) | 0 | 2 (0.5) | 0 | |
| Pericardial leak | 13 (2.3) | 4 (3.6) | 8 (2.2) | 1 (1.2) | .531 |
| Death during hospital admission | 14 (2.5) | 0 | 11 (3.0) | 3 (3.6) | .041 |
BARC, Bleeding Academic Research Consortium; DMR, degenerative mitral regurgitation; FMR, functional mitral regurgitation; MMR, mixed mitral regurgitation;
Data are expressed as No. (%).
There were 14 in-hospital deaths: 11 patients had FMR and 3 had MMR. The causes of the in-hospital mortality in the FMR group were as follows: 7 due to refractory HF, 2 due to sepsis (1 of urinary origin and another of respiratory origin), 1 stroke, and 1 multiorgan failure. In the MMR group, the causes were refractory HF in 2 and respiratory failure in 1. There were no in-hospital deaths in the DMR group.
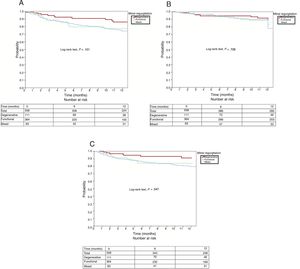
Primary endpointAt 12 months of follow-up, the total number of events of the primary study endpoint was 95 (18.9%) in the entire series: 11 in the DMR group (11.3%), 71 in the FMR group (21.3%), and 13 in the MMR group (18.1%), with no significant differences among the 3 groups (P=.101). All-cause mortality in the entire series at 12 months of follow-up was 14% (78 patients), distributed according to MR type as follows: 13 patients in the DMR group (11.7%), 57 in the FMR group (15.7%), and 8 in the MMR group (9.6%), with no significant differences among the 3 groups (P=.728). The percentage of readmissions for HF in the entire series at 12 months was 18% (101 patients): 13 in the DMR group (11.7%), 77 in the FMR group (21.2%), and 11 in the MMR group (13.3%); the rate was significantly higher in the FMR group (P=.047). Figure 1 shows the 12-month survival curves for the composite endpoint (figure 1A), all-cause mortality (figure 1B), and readmissions for HF (figure 1C), according to MR cause.
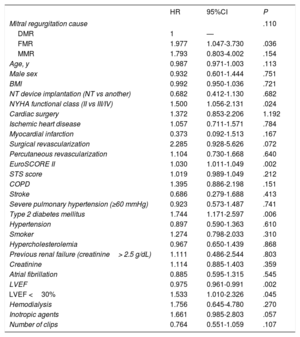
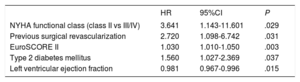
Univariate and multivariate analyses of the composite endpoint at 12 months are shown in table 4 and table 5, respectively. The variables associated with the primary endpoint were functional class (P=.029), previous surgical revascularization (P=.031), the EuroSCORE II (P=.003), diabetes mellitus (P=.037), and left ventricular ejection fraction (P=.015). Follow-up was complete for 79.93% of the sample.
Univariable analysis of the primary study endpoint (death or readmission at 12 months)
| HR | 95%CI | P | |
|---|---|---|---|
| Mitral regurgitation cause | .110 | ||
| DMR | 1 | — | |
| FMR | 1.977 | 1.047-3.730 | .036 |
| MMR | 1.793 | 0.803-4.002 | .154 |
| Age, y | 0.987 | 0.971-1.003 | .113 |
| Male sex | 0.932 | 0.601-1.444 | .751 |
| BMI | 0.992 | 0.950-1.036 | .721 |
| NT device implantation (NT vs another) | 0.682 | 0.412-1.130 | .682 |
| NYHA functional class (II vs III/IV) | 1.500 | 1.056-2.131 | .024 |
| Cardiac surgery | 1.372 | 0.853-2.206 | 1.192 |
| Ischemic heart disease | 1.057 | 0.711-1.571 | .784 |
| Myocardial infarction | 0.373 | 0.092-1.513 | .167 |
| Surgical revascularization | 2.285 | 0.928-5.626 | .072 |
| Percutaneous revascularization | 1.104 | 0.730-1.668 | .640 |
| EuroSCORE II | 1.030 | 1.011-1.049 | .002 |
| STS score | 1.019 | 0.989-1.049 | .212 |
| COPD | 1.395 | 0.886-2.198 | .151 |
| Stroke | 0.686 | 0.279-1.688 | .413 |
| Severe pulmonary hypertension (≥60 mmHg) | 0.923 | 0.573-1.487 | .741 |
| Type 2 diabetes mellitus | 1.744 | 1.171-2.597 | .006 |
| Hypertension | 0.897 | 0.590-1.363 | .610 |
| Smoker | 1.274 | 0.798-2.033 | .310 |
| Hypercholesterolemia | 0.967 | 0.650-1.439 | .868 |
| Previous renal failure (creatinine> 2.5 g/dL) | 1.111 | 0.486-2.544 | .803 |
| Creatinine | 1.114 | 0.885-1.403 | .359 |
| Atrial fibrillation | 0.885 | 0.595-1.315 | .545 |
| LVEF | 0.975 | 0.961-0.991 | .002 |
| LVEF <30% | 1.533 | 1.010-2.326 | .045 |
| Hemodialysis | 1.756 | 0.645-4.780 | .270 |
| Inotropic agents | 1.661 | 0.985-2.803 | .057 |
| Number of clips | 0.764 | 0.551-1.059 | .107 |
95%CI, 95% confidence interval; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DMR, degenerative mitral regurgitation; FMR, functional mitral regurgitation; HR, hazard ratio; LVEF, left ventricular ejection fraction; MMR, mixed mitral regurgitation; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons.
Multivariable analysis of the primary study endpoint (death or readmission at 12 months)
| HR | 95%CI | P | |
|---|---|---|---|
| NYHA functional class (class II vs III/IV) | 3.641 | 1.143-11.601 | .029 |
| Previous surgical revascularization | 2.720 | 1.098-6.742 | .031 |
| EuroSCORE II | 1.030 | 1.010-1.050 | .003 |
| Type 2 diabetes mellitus | 1.560 | 1.027-2.369 | .037 |
| Left ventricular ejection fraction | 0.981 | 0.967-0.996 | .015 |
95%CI, 95% confidence interval; HR, hazard ratio; NYHA, New York Heart Association.
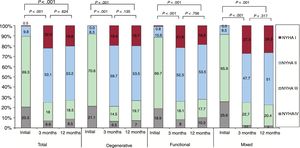
Regarding the changes in the NYHA functional class (figure 2), there was a clear improvement at 3 months. This was maintained at 1 year, with 75.1% of the patients in NYHA I or II. This improvement was evident for all etiological groups of MR. The changes over time in the MR grade are shown in figure 3. There was a significant reduction in MR grade at 3 months, which was maintained at 12 months. At the end of follow-up, 73.2% of the patients had ≤ grade II MR. This improvement was found in all etiological groups of MR.
This collaborative project of 16 Spanish hospitals was performed in accordance with the recommendations of the Spanish Society of Cardiology regarding TMVR using a registry sponsored by this body and with real-life data from patients with symptomatic severe MR treated by TMVR with MitraClip.
Our series shows the safety and efficacy of TMVR with MitraClip in a high-risk population, with a persistent improvement in functional class and a reduction in MR grade. There were no significant differences in the primary study endpoint among the different MR types, although there was a trend toward a worse prognosis for FMR and MMR than for DMR.
In our series, the percentage of patients with FMR was higher than that of DMR. These data are in line with those published by other European registries (71% in TRAMI,9 77% in ACCES-EU,10 76% in GRASP,11 and 72% in Sentinel12), reflecting the widespread use in Europe of this treatment for severe FMR (approximately 2-thirds with FMR vs 1-third with DMR). This contrasts with results from the United States, where TMVR has thus far been used predominantly for DMR.6–8
Our patients’ clinical profile was similar to that reported in previous European series. The DMR group was older, whereas the FMR group had higher percentages of men, ischemic heart disease, percutaneous revascularization, and chronic obstructive pulmonary disease.9–12
We believe that it is important to highlight the high overall success of the procedure (94%), which was similar for the different types of MR. The procedural success rate was higher than that published in the first trial (EVEREST II8) but comparable to the results of other registries (97%,9 91%,10 100%,11 and 95%12). Together with the low rate of major procedure-related complications, this success rate reflects the applicability of the previous evidence to our setting.9–12 The accumulation of greater experience by the teams performing the procedure has been key to the improved results.
In our series, there were no significant differences in the number of clips implanted according to MR type. In previous series, a higher number of clips were indicated for the treatment of DMR with larger regurgitant volumes, although adequate treatment has also been documented of severe MR with a single clip.16 Proper patient selection is essential to correctly determine the number of clips to use.17,18
The MR reduction after implantation in our series was significant for both DMR and FMR, in line with the results of the main clinical trials7,13,14 and international registries.9–12 Our data show that the MR reduction is maintained at ≤ grade II in 73.2% of patients at 12 months. This percentage is consistent with that reported in the first European registry10 and in more recent ones.11
The composite event-free survival of the entire series was 77.1%, with no significant differences (P=.101) among the different groups: 85.8% for DMR, 74.5% for FMR, and 77.7% for MMR.
The absence of differences between the DMR and FMR groups for this composite endpoint was also reported by Nickenig et al.12 and, as in our series, they found a tendency for worse prognosis with FMR. In our work, the primary outcome event rate was higher in the FMR group (21.3%) than in the DMR group (11.3%). This result should be interpreted in the context of the sample size and the length of follow-up in this study; larger studies with a longer follow-up might find significant differences.
This trend could be explained by the presence of a higher risk profile in the FMR group than in the DMR group (ie, more comorbidities and worse left ventricular ejection fraction). Although there was a certain tendency for worse prognosis in the FMR and MMR groups vs the DMR group, there was no significant difference in the composite endpoint.
The all-cause mortality at 1 year of follow-up in our series is very similar to that of some recent European registries (15.3%12 and 14.4%11) and somewhat lower than that of others from the same region (19.2%10 and 20.3%9), probably due to the different clinical characteristics of the patients included.
Our data are in line with the findings of a recent meta-analysis that identified an overall all-cause mortality rate of 16%, with no difference between DMR and FMR (HR=1.26; 95%CI, 0.90-1.77; P=.18). This work also showed the tendency of the FMR group to have a worse prognosis and a higher percentage of admissions for HF.19
According to MR cause, all-cause mortality per year was 11.7% in the DMR group of our series. This figure is slightly higher than that reported in the EVEREST II trial7 because it included low-risk patients who were surgical candidates.
Regarding FMR, all-cause mortality at 1 year was 15.7% in our series. This rate is lower than that published recently in the 2 main clinical trials comparing TMVR with medical therapy alone. One of the possible explanations for this difference is the higher risk characteristics of the patients included in these studies. In the COAPT trial,13 the mean Society of Thoracic Surgeons (STS) score was 7.8% in the MitraClip group, with more than 40% of the patients in this group having an STS ≥ 8, whereas the median was 3.3 in the FMR group in our series. Similarly, the average EuroSCORE II in the MitraClip group was 6.6 in the MITRA-FR trial,14 but 4.4 in the FMR group in our series.
This work presents the data related to MitraClip treatment; however, there are other percutaneous mitral repair devices on the market with less accumulated experience, that act on different therapeutic targets of the mitral valve apparatus, and that can even complement the treatment with this MitraClip.
LimitationsThis work has some limitations. The number of patients comprising this series is small and the follow-up is not particularly long. Because this is an observational study, there is no evidence that it has sufficient power to detect significant differences in the primary endpoint; in addition, the different distributions of some variables among the 3 groups could introduce biases during follow-up. The classification of some patients as having MMR is controversial and this approach could represent the loss of a percentage, albeit small, of patients from the other 2 subgroups. Finally, another limitation may be the absence of a centralized imaging laboratory.
CONCLUSIONSThis is the largest reported series of patients with MR treated by TMVR in Spain and reflects the general use of the technique in our environment. This work confirms, through real-life data from Spain, the safety and efficacy of the treatment and documents the main factors associated with prognosis during the first year of follow-up of these patients.
CONFLICTS OF INTERESTNone declared.
- –
TMVR with the MitraClip device is safe and effective for both degenerative and functional MR. No significant differences have been found in the prognosis of patients treated with TMVR according to MR cause.
- –
This article contributes to a better understanding of the setting of severe and symptomatic MR treated by TMVR according to cause. This work confirms the safety and efficacy of the treatment in Spain and documents the main factors associated with prognosis during the first year of follow-up of these patients.
.