Keywords
INTRODUCTION
The development of drug-eluting stent (DES) therapy has led to a significant reduction in both angiographic in-stent restenosis and clinical target lesion revascularisation when compared with percutaneous intervention using bare metal stents (BMS).1,2 Initial studies with DES suggested re-intervention rates below 10%,≥,4 though with the passage of time it has become clear that patients with more complex disease patterns and those in a real world setting have significantly higher rates of target vessel failure.5-11 Furthermore, the widespread adoption of DES implantation has led to a significant absolute number of patients presenting with DES restenosis with perhaps in the region of 200 000 cases per annum in the United States alone. Consequently the magnitude of this clinical entity is significant and its optimal treatment remains a challenge, particularly in those with diffuse pattern restenosis.
Recent trial data suggests that implantation of a DES seems superior to intravascular brachytherapy, at least as far as BMS restenosis is concerned.12,13 In reports of cases of DES restenosis, it remains unclear whether adopting a strategy of using a different DES-type to that originally implanted (so called "hetero" DES treatment) is superior to treatment with the same DES-type (so-called "homo" DES treatment).14-17 Sirolimus-eluting stents (SES) have been shown to offer superior angiographic and clinical outcomes to paclitaxel-eluting stents (PES) in a number of sub-sets of patients with high-risk clinical features including those with baremetal stent in-stent restenosis.2,18-23 We therefore studied angiographic and clinical outcomes of patients with PES-restenosis treated exclusively with SES.
METHODS
Study Population
In October 2004, our institution adopted a policy of using SES (Cypher stent) as the device of choice in patients presenting with PES (Taxus stent) treatment failure. Patients treated in this manner between October 2004 and July 2005 were enrolled in the current study. Inclusion criteria were in-stent restenosis, defined as luminal re-narrowing ≥50% within the stented segment or within a 5 mm segment either proximal or distal to the stent edges, and either symptoms or signs of recurrent ischaemia or stenosis ≥70% diameter. Patients with acute stent thrombosis as a cause of luminal re-narrowing were excluded. The complexity of the restenotic lesions was classified according to the classification reported by Mehran et al.24 All patients provided written informed consent for the study procedure and subsequent data collection and analysis for research purposes. Patients were prescribed clopidogrel post procedure for a minimum of 1 year. Clinical follow up was performed at 1, 6, 12, and 24 months. All patients were asked to return for coronary angiography 6-8 months after treatment of their in-stent restenosis.
Quantitative Coronary Angiography Evaluation and Definitions
Baseline, post procedural, and follow-up coronary angiograms were digitally recorded and assessed off-line in the quantitative angiographic core laboratory (Deutsches Herzzentrum München) with an automated edge-detection system (CMS version 6.0, Medis Medical Imaging Systems) by 2 independent experienced operators unaware of either the initial stent implantation or the subsequent stent used in the treatment of the restenotic lesion. All measurements were performed on cineangiograms recorded after the intracoronary administration of nitroglycerin. The same single worst-view projection was used at all times. The contrast-filled non-tapered catheter tip was used for calibration. The quantitative parameters that were measured included reference diameter of the vessel, minimal diameter of the lumen pre- and post-procedure, diameter stenosis, and late lumen loss (difference between minimal luminal diameter at the end of the procedure and minimal luminal diameter at follow-up). In addition, quantitative analysis was performed on the "in-segment" area, including the stented segment, as well as both 5-mm margins proximal and distal to the stent. The procedure was considered successful if residual stenosis was <30% with TIMI flow grade 3. Binary angiographic restenosis was defined as diameter stenosis ≥50% in the in-segment area. Target lesion revascularisation (TLR; clinical restenosis) was defined as any revascularisation procedure percutaneous or surgical, involving the target lesion and accompanied by symptoms or signs of ischaemia. A major adverse cardiac event (MACE) during follow-up was defined as death, myocardial infarction, or target lesion revascularisation.
Statistical Analysis
Continuous variables were expressed as mean (standard deviation); categorical variables, as number (percentages). Analyses were performed with the S-Plus statistical package (Mathsoft Inc, Seattle, Wash).
RESULTS
Between October 2004 and July 2005, we identified 43 consecutive patients (43 lesions) with in-stent restenosis in a PES who underwent clinically driven revascularisation with SES implantation. The baseline patient and angiographic characteristics are shown in Table 1. The majority of patients had complex multi-vessel coronary disease. As expected, there were a significant proportion of patients with diabetes (34.8%). Approximately one quarter of lesions involved a coronary bifurcation. A focal pattern of restenosis was seen in 33 lesions (76.7%) whereas diffuse restenosis was observed in the remainder (diffuse in-stent, 8 [19%]; diffuse beyond stent, 1 [2%]; diffuse occlusive, 1 [2%]). Procedural success was 100%. Procedural characteristics are shown in Table 2. Early complications were few; there was 1 death at 30 days.
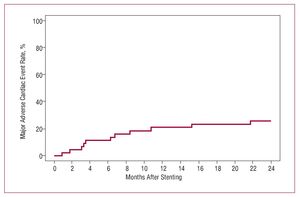
Angiographic follow-up was available for 36 patients (83.7%). Binary restenosis was found in 6 patients (16.7%). Table 2 includes results of quantitative coronary angiography analysis at 6-month follow up. In-stent late luminal loss was 0.32 (0.54) mm. Clinical outcome data at 30 days and 2 years were available for all patients and are displayed in Table 3. There were 2 deaths during follow up. Five patients (11.6%) required repeat percutaneous revascularisation of the stented segment and 2 patients (4.7%) underwent subsequent coronary bypass surgery, giving an overall TLR rate of 16.3%. At 2 years, 74.2% of patients remained free of MACE (Figure).
Figure. Cumulative major adverse cardiac event (MACE) rate at 2 years. MACE is defined as death, myocardial infarction, or target lesion revascularisation.
DISCUSSION
The salient findings of this study are that: a) treatment of PES restenosis with SES is feasible and the implantation of a second different DES was not associated with any indication of adverse effects; b) luminal gain is well preserved at angiographic re-study; c) 2 year TLR and MACE rates remain significant.
There remains a paucity of published data on many aspects surrounding the most appropriate management of DES treatment failure.25 While previous research has shown that in BMS treatment failure, DES implantation is superior to both balloon angioplasty18,26 and intravascular brachytherapy,12,13 it is unclear whether findings from the treatment of BMS restenosis may be extrapolated to the management of DES treatment failure. If we examine the mechanisms proposed to account for the superiority of DES over brachytherapy in recent trials on patients with in-stent restenosis12,13 (greater initial acute gain, comparable late preservation of gain, and mitigation of the radiation-associated edge restenosis effect) then it seems a reasonable therapeutic approach to use another DES, at least initially, in the management of DES restenosis. At present there is no data to support the dominance of one drug eluting stent over another in this population, or indeed a strategy of same or "homo" DES over different or "hetero" DES.15-17
Our study is unique in reporting outcomes in patients undergoing a pre-specified standardised treatment (namely SES implantation) for DES restenosis and including a high proportion of patients with angiographic follow-up. In this regard, the relatively low rate of angiographic restenosis observed (16.7%) is noteworthy. Despite similar or larger post-intervention reference vessel sizes, Lemos et al14 and
Cosgrave et al16 reported binary restenosis rates of 42% and 26.4% respectively in groups of patients that seemed well matched in terms of disease complexity. The former group also reports a late luminal loss which approaches that seen in the BMS era. In both studies, patients with restenosis were treated either with PES or SES and device-specific outcomes are not described. It may be argued that the higher rates of recurrent restenosis are due to a dilution of the DES treatment effect by the inclusion of PES as well as SES in the treatment protocols, and its corollary that device-specific differences in restenotic efficacy may be of particular importance in this group of complex patients. This may be interpreted as reaffirmation of the central tenet of percutaneous coronary intervention in the DES era, namely that durability of procedural results is related to both optimization of acute procedural gain and minimization of late loss (eg, the use of SES), regardless of whether the lesions are de novo or restenotic.27,28
On the other hand, our study reemphasizes that percutaneous revascularisation of DES restenosis remains associated with relatively significant rates of MACE and TLR at 2 years (25.8% and 16.3% respectively), though this may be seen as acceptable in a high risk cohort patients with challenging coronary disease complexity. This is in keeping with observations that in-stent restenosis, as opposed to restenosis following angioplasty, is not a benign process and may be associated with poor outcomes in the medium term.26,29 Interestingly, these events occurred progressively over the time course of follow-up with no clear signal of influence from protocol mandated 6-8 month re-angiography. It should be noted that these figures are higher than rates reported in the use of SES to treat BMS restenosis at our centre18 (11.0% and 8.0% respectively) despite an apparently similar disease complexity (diffuse pattern restenosis in 40%, median vessel size [interquartile range] 2.60 mm [2.23-2.93 mm], median lesion length [interquartile range] 12.4 mm [7.9-18.3 mm]). Although the validity of comparisons is limited by the small numbers involved and the additional 12 months of clinical follow up in this current study, DES treatment failure likely represents a more recalcitrant disease process. The hypothesis that patients with DES restenosis, particularly those with diffuse pattern restenosis, represent a sub-population with characteristics (including polymer hypersensitivity and anti-proliferative/immunosuppressive therapy resistance) predisposing to failure of a repeat DES implantation approach, and its corollary, that the adoption of an alternative treatment strategy (ie, brachytherapy or surgical revascularisation) might be superior, is an important consideration and awaits further study.30
Study Limitations
Our observations are limited by the small number of patients studied. As a result meaningful multivariate analysis of the factors predisposing to recurrent DES failure (or so-called multi-drug resistant in-stent restenosis) was precluded. We do not have data for comparison on the outcomes of a similar cohort of patients with PES restenosis who were treated with repeated PES implantation as we deemed this a priori a suboptimal therapeutic strategy.
CONCLUSION
In conclusion, we have demonstrated that in cases of PES treatment failure, SES implantation is a reasonable therapeutic strategy associated with durable luminal gain. Two-year TLR and MACE rates are significant but acceptable in this cohort of patients with a complex disease process. Further data from randomized trials is awaited in order to ascertain whether there exists a device-specificity in the use of DES to treat previous DES failure and on the optimal management of patients with recalcitrant multi-drug resistant restenosis.
ABBREVIATIONS
BMS: bare metal stent
DES: drug-eluting stent
MACE: major adverse cardiac event PES: paclitaxel-eluting stent
SES: sirolimus-eluting stent
TLR: target lesion revascularization
SEE EDITORIAL ON PAGEs 1120-2
Robert Byrne is supported by a training grant from the Irish Board for Training in Cardiovascular Medicine which is sponsored by A. Menarini Pharmaceuticals (Ireland).
Correspondence: R. Byrne, MB MRCPI,
ISAR Research Centre,
Lazarettstrasse 36. D-80636 München, Germany
E-mail: byrne@dhm.mhn.de
Received February 5, 2008.
Accepted for publication June 9, 2008.