Multiple long-term prognostic clinical and procedural factors and several prognostic biomarker factors have been identified in ST-segment elevation myocardial infarction. However, current risk prediction models derived from these factors only provide a rough estimate of individual risk and more efforts are required to improve prognosis prediction.
A technique based on proximity extension assays allows analysis of a large number of cardiovascular and inflammation-related proteins present in plasma at concentrations <10ng/mL at the same time.
We used, in an exploratory way, a proximity extension assay approach with the aim of identifying potential biomarkers, previously selected because of a known or potential relationship with cardiovascular or inflammation, successful reperfusion and long-term prognosis in a selected ST-segment elevation myocardial infarction cohort.
This study included consecutive patients with ST-segment elevation myocardial infarction undergoing primary angioplasty in the University Clinic Hospital in Valencia, Spain, as described elsewhere.1 Using proximity extension assay technology, 184 proteins included in Olink's inflammation and cardiovascular diseases-III panels were analyzed. The primary endpoint was long-term all-cause mortality. Secondary endpoints were a composite of all-cause mortality and spontaneous nonfatal myocardial infarction (major adverse cardiac events [MACE]), lack of ST-segment resolution (≥ 50%) at 60minutes, and the absence of TIMI 3 flow at the end of the procedure. Clinical follow-up was terminated 5 years after the inclusion period.
Of a total of 116 patients, plasma was available from 90 patients and, after exclusion of controls, only 84 patients could be analyzed for each panel. The measures are expressed in an arbitrary unit as log2 of the concentration values because of nonnormal distribution. Peripheral and coronary samples were obtained at almost the same time and a principal components analysis was performed, showing what could be considered as biological replicates.
A univariate analysis was performed to identify biomarkers that were related to the endpoints (P <.10). Three models were built and sequentially applied for each endpoint and each identified biomarker including: model 1, age and sex; model 2, model 1 plus all endpoint-related variables (P <.10 in the univariate analysis); model 3, model 2 plus variables considered clinically relevant by the investigators (age, sex, diabetes mellitus time from symptoms to reperfusion, culprit vessel diameter, hypertension, hypercholesterolemia, smoking, troponin T, previous coronary revascularization, left ventricular ejection fraction at the time of discharge). Candidate biomarkers were excluded for the next model if the P value was>.05 in either of the 2 replicates.
ST-segment resolution ≥ 50% was achieved in 53 (63.1%) patients and TIMI 3 flow was observed in 61 (81.0%) patients. Long-term follow-up was completed at a median of 4.14 [3.73-4.49] years with an overall mortality of 9.5% (n=8), and there were 5 (6.0%) nonfatal myocardial infarctions (MI), obtaining a MACE rate of 14.3%.
No significant differences were found related to ST-segment resolution or TIMI 3 and consequently multivariate analyses were not performed for these endpoints.
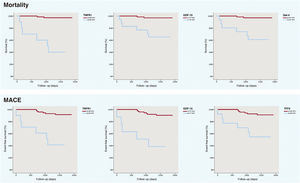
After Benjamini-Hochberg correction, 9 biomarkers were selected as candidates to be predictors for long-term mortality in model 1. Only 4 remained as significant in model 2 and galectin-4 (Gal-4) (hazard ratio [HR] 2.998; 95% confidence interval [95%CI] 1.489-5.996; P=.002), growth/differentiation factor 15 (GDF-15) (HR, 2.483; 95%CI, 1.072-5.753; P=.034) and tumor necrosis factor receptor 1 (TNFR1) (HR, 10.554; 95%CI, 2.950-37.767; P=.0003) in model 3 with similar values in the same analyses with the biological replicates (table 1). For MACE, all the initial candidate biomarkers remained as significant predictors in the 3 models (GDF-15, trefoil factor 3 [TFF3] and TNFR1) (table 1). Kaplan-Meier plots were generated to express graphically the association of the predictors with mortality and MACE (figure 1) and C-statistics for model 3 for mortality and MACE are shown in table 1. The cutoff values used derived from receiver operating characteristic curves (5.331 for Gal-4, 8.365 for TNFR1 and 7.727 for GDF-15 [mortality] and 6.3620 for TFF3, 8.365 for TNFR1 and 8.477 for GDF-15 [MACE]).
Multivariate analyses for mortality and MACE
| Mortality | Cox1 (P value) | Cox2 (P value) | Cox3 (P value) | Cox 3 HR [95%CI] | ||||
|---|---|---|---|---|---|---|---|---|
| Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | |
| Gal-4 | .016179 | .004875 | .002077 | .00082 | .002077 | .005476 | 2.998 [1.489-5.996] | 3.3378 [1.431-7.976] |
| GDF15 | .000016 | .000565 | .000276 | .000131 | .022067 | .000131 | 2.483 [1.072-5.753] | 2.644 [1.607-4.352] |
| TNFR1 | .000038 | .000039 | .000291 | .000139 | .000291 | .000139 | 10.554 [2.950-37.767] | 36.653 [5.747-233.735] |
| FABP4 | .010321 | .000174 | .000641 | .000511 | NS | NS | ||
| IGFBP2 | .031316 | .034437 | .088723 | .037646 | ||||
| TFF3 | .000054 | .000046 | NS | .000144 | ||||
| TGF-α | .000241 | .003828 | NS | .002841 | ||||
| TNFR2 | .001752 | .01811 | NS | NS | ||||
| LTBR | .000862 | .029051 | NS | NS | ||||
| MACE | Cox 1 (P value) | Cox 2 (P value) | Cox 3 (P value) | Cox 3 HR [95%CI] | ||||
|---|---|---|---|---|---|---|---|---|
| Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | |
| TFF3 | .001565 | .000278 | .001565 | .000278 | .017755 | .002164 | 6.472 [1.995-21.424] | 12.192 [2.913-57.233] |
| GDF-15 | .000282 | .000026 | .000282 | .000026 | .000418 | .000091 | 2.140 [1.398-3.277] | 2.658 [1.615-4.373] |
| TNFR1 | .001561 | .000097 | .0036 | .000097 | .000708 | .000134 | 5.908 [2.296-15.205] | 34.406 [5.890-200.961] |
| C-statistic | Long-term mortality | MACE |
|---|---|---|
| Clinical | 0.770 | 0.730 |
| TNFR1 | 0.918 | 0.778 |
| GDF-15 | 0.832 | 0.717 |
| Gal-4 | 0.817 | NA |
| TFF3 | NA | 0.793 |
95%CI, 95% confidence interval; FABP4, fatty acid-binding protein 4; Gal-4, galectin-4; GDF-15, growth/differentiation factor 15; HR, hazard ratio; IGFBP2, insulin-like growth factor binding protein 2; LTBR, lymphotoxin beta receptor; MACE, major adverse cardiac events; NA, not applicable; NS, not significant; TFF3, trefoil factor 3; TGF-α, transforming growth factor-α; TNFR1, tumor necrosis factor receptor 1; TNFR2, tumor necrosis factor receptor 2.
Cox 1 model: age and sex. Cox 2 model: age, sex, diabetes mellitus time from symptoms to reperfusion and culprit vessel diameter. Cox 3 model: age, sex, diabetes mellitus time from symptoms to reperfusion, culprit vessel diameter, hypertension, hypercholesterolemia, smoking, troponin T, previous coronary revascularization, left ventricular ejection fraction at the time of discharge.
HR and 95%CI are only shown for the Cox 3 model. P values are shown for all the models.
Replicate 1 reflects peripheral plasma and replicate 2 coronary plasma.
This study identifies 2 new potential long-term prognostic biomarkers in MI: Gal-4 for overall mortality and TFF3 for MACE. Gal-4 is a carbohydrate-binding protein belonging to the galectin family that, as far as we know, has not been related to cardiovascular diseases. However, it has been described that Gal-4 increases interleukin 62 secretion, which has been demonstrated to be deleterious in MI. TFF3 has been found to a be a prognostic biomarker in chronic heart failure3 but its mechanism of action in MI is still unknown.
Skau et al.4 performed a similar study with Olink's cardiovascular diseases-I panel, identifying GDF-15 and TRAIL-R2 as long-term prognostic biomarkers but Gal-4 and TFF3 were not studied and TRAIL-R2 was not included in either our cardiovascular diseases-III or inflammation panels.
TNFR15 and GDF-156 are 2 well-known and studied prognostic biomarkers in MI. Despite our low number of events, these biomarkers and Gal-4 and TFF3, which have been tested in a robust method intended to minimize false results, could be strong prognostic biomarker candidates in MI and larger studies should be performed.
FUNDINGThis work was supported by grants from Spain's Ministry of Economy and Competitiveness through the Carlos III Health Institute: RD12/0042/0010 and CB16/11/00420; ERDF (European Regional Development Fund); Health Research Fund.
CONFLICTS OF INTERESTJ. Núñez reports personal fees from Novartis, personal fees from Vifor, personal fees from Boehringuer Ingelheim, outside the submitted work. All other authors declare no conflicts of interest.