In the last few years, the increasing clinical importance of functional tricuspid regurgitation (TR) has contributed to the growing interest in the early diagnosis and treatment of this disease, which has historically been neglected. TR has a high prevalence and is associated with adverse prognosis, since the presence of moderate or severe TR is associated with increased morbidity and mortality per se. Nevertheless, isolated TR surgery continues to have high operative morbidity and mortality and prolonged hospitalizations, and a recent propensity-score matching study demonstrated that surgery was not associated with improved long-term survival compared with medical therapy alone.1 In this context, the initial experiences of transcatheter techniques for the treatment of TR have demonstrated feasibility and safety, with promising hemodynamic and clinical results.
TRICENTO transcatheter heart valve (TTHV) (NVT AG, Muri, Switzerland) aims to abolish the systolic backflow in the superior and inferior caval veins, although it does not directly act upon the TR. TTHV is composed of a covered nitinol stent with a lateral bicuspid valve component made of porcine pericardium. Since the first-in-man implantation in 2018,2 there have only been reports of very limited initial single-center experiences with short-term follow-up results.3,4
A total of 6 consecutive patients underwent TTHV implantation at 4 participating centers in Spain between November 2018 and August 2019. The baseline and procedural characteristics are displayed in table 1. All patients had congestive heart failure (HF) and New York Heart Association (NYHA) class III-IV in relation to severe functional TR despite optimal medical treatment. All patients showed dilatation of the inferior vena cava and holosystolic backflow in the hepatic veins. Heart Team discussion deemed them inappropriate candidates for open heart surgery. Baseline transthoracic echocardiogram, right heart catheterization, and multislice computed tomography (MSCT) were performed in all patients. Patients with tricuspid annular plane systolic excursion (TAPSE) ≤ 13mmHg, left ventricular ejection fraction ≤ 30%, systolic pulmonary artery pressure> 70mmHg or unfavorable anatomy by MSCT were excluded according to manufacturer recommendations. The study was conducted in accordance with the principles of the Declaration of Helsinki.
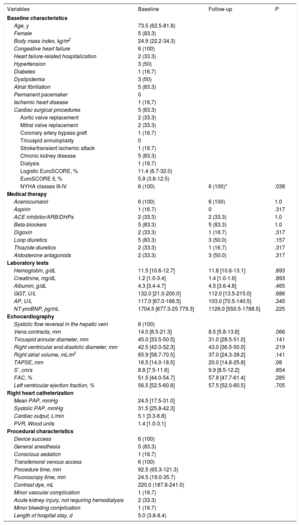
Clinical, echocardiographic and procedural characteristics of patients undergoing TTHV implantation.
| Variables | Baseline | Follow-up | P |
|---|---|---|---|
| Baseline characteristics | |||
| Age, y | 73.5 (62.5-81.8) | ||
| Female | 5 (83.3) | ||
| Body mass index, kg/m2 | 24.9 (22.2-34.3) | ||
| Congestive heart failure | 6 (100) | ||
| Heart failure-related hospitalization | 2 (33.3) | ||
| Hypertension | 3 (50) | ||
| Diabetes | 1 (16.7) | ||
| Dyslipidemia | 3 (50) | ||
| Atrial fibrillation | 5 (83.3) | ||
| Permanent pacemaker | 0 | ||
| Ischemic heart disease | 1 (16,7) | ||
| Cardiac surgical procedures | 5 (83.3) | ||
| Aortic valve replacement | 2 (33.3) | ||
| Mitral valve replacement | 2 (33.3) | ||
| Coronary artery bypass graft | 1 (16.7) | ||
| Tricuspid annuloplasty | 0 | ||
| Stroke/transient ischemic attack | 1 (16.7) | ||
| Chronic kidney disease | 5 (83.3) | ||
| Dialysis | 1 (16.7) | ||
| Logistic EuroSCORE, % | 11.4 (6.7-32.0) | ||
| EuroSCORE II, % | 5.9 (3.6-12.5) | ||
| NYHA classes III-IV | 6 (100) | 6 (100)* | .038 |
| Medical therapy | |||
| Acenocumarol | 6 (100) | 6 (100) | 1.0 |
| Aspirin | 1 (16.7) | 0 | .317 |
| ACE inhibitor/ARB/DHPs | 2 (33.3) | 2 (33.3) | 1.0 |
| Beta-blockers | 5 (83.3) | 5 (83.3) | 1.0 |
| Digoxin | 2 (33.3) | 1 (16.7) | .317 |
| Loop diuretics | 5 (83.3) | 3 (50.0) | .157 |
| Thiazide diuretics | 2 (33.3) | 1 (16.7) | .317 |
| Aldosterone antagonists | 2 (33.3) | 3 (50.0) | .317 |
| Laboratory tests | |||
| Hemoglobin, g/dL | 11.5 [10.6-12.7] | 11.8 [10.6-13.1] | .893 |
| Creatinine, mg/dL | 1.2 [1.0-3.4] | 1.4 [1.0-1.6] | .893 |
| Albumin, g/dL | 4.3 [3.4-4.7] | 4.5 [3.6-4.8] | .465 |
| GGT, U/L | 132.0 [21.0-200.0] | 112.0 [13.5-215.0] | .686 |
| AP, U/L | 117.0 [67.0-166.5] | 103.0 [70.5-140.5] | .345 |
| NT-proBNP, pg/mL | 1704.5 [677.3-25 779.3] | 1126.0 [550.5-1788.5] | .225 |
| Echocardiography | |||
| Systolic flow reversal in the hepatic vein | 6 (100) | ||
| Vena contracta, mm | 14.0 [8.5-21.3] | 8.5 [5.8-13.8] | .066 |
| Tricuspid annular diameter, mm | 45.0 [33.5-50.5] | 31.0 [28.5-51.0] | .141 |
| Right ventricular end-diastolic diameter, mm | 42.5 [42.0-52.3] | 43.0 [36.5-50.0] | .219 |
| Right atrial volume, mL/m2 | 65.9 [58.7-70.5] | 37.0 [24.3-39.2] | .141 |
| TAPSE, mm | 16.5 [14.0-18.5] | 20.0 [14.8-25.8] | .08 |
| S’, cm/s | 8.8 [7.5-11.6] | 9.9 [8.5-12.2] | .854 |
| FAC, % | 51.5 [44.0-54.7] | 57.8 [47.7-61.4] | .285 |
| Left ventricular ejection fraction, % | 56.5 [52.5-60.8] | 57.5 [52.0-60.5] | .705 |
| Right heart catheterization | |||
| Mean PAP, mmHg | 24.5 [17.5-31.0] | ||
| Systolic PAP, mmHg | 31.5 [25.8-42.3] | ||
| Cardiac output, L/min | 5.1 [3.3-6.8] | ||
| PVR, Wood units | 1.4 [1.0-3.1] | ||
| Procedural characteristics | |||
| Device success | 6 (100) | ||
| General anesthesia | 5 (83.3) | ||
| Conscious sedation | 1 (16.7) | ||
| Transfemoral venous access | 6 (100) | ||
| Procedure time, min | 92.5 (65.3-121.3) | ||
| Fluoroscopy time, min | 24.5 (19.0-35.7) | ||
| Contrast dye, mL | 220.0 (187.8-241.0) | ||
| Minor vascular complication | 1 (16.7) | ||
| Acute kidney injury, not requiring hemodialysis | 2 (33.3) | ||
| Minor bleeding complication | 1 (16.7) | ||
| Length of hospital stay, d | 5.0 (3.8-8.4) | ||
ACE inhibitor, angiotensin-converting enzyme inhibitors; AP, alkaline phosphatase; ARB, angiotensin receptor blocker; DHPs, dihydropyridine calcium channel blockers; EuroSCORE, The European System for Cardiac Operative Risk Evaluation; FAC, fractional area change; GGT, γ-glutamyltransferase; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association functional classification; PAP, pulmonary artery pressure; PVR, pulmonary vascular resistance; S’, tissue Doppler–derived tricuspid lateral annular systolic velocity; TAPSE, tricuspid annular plane systolic excursion.
Continuous variables are reported as median [25th-75th percentile] and categorical variables are presented as No. (%).
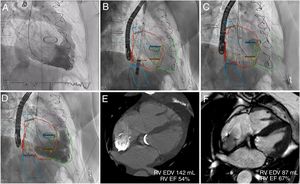
TTHV procedures were performed under general anesthesia (n=5) or conscious sedation (n=1) with intraprocedural imaging guidance using transoesophageal echocardiography and fluoroscopy. Three-dimensional MSCT-fluoroscopy fusion imaging was successfully used in 2 patients (figure 1). Baseline right ventriculography was performed and a deflectable catheter marked the junction of the inferior vena cava and right atrium (RA). The TTHV delivery system (24-Fr) was advanced from the right femoral vein. Device implantation was performed by gradual and controlled top-down deployment after the distal radiopaque marker was aligned to achieve correct valvular component orientation. Final right ventriculography showed competence of the bicaval valve system, without reversed flow in the suprahepatic veins, absence of leaks or obstruction of the hepatic veins. The device was successfully deployed in 100% of the patients (figure 1). Patients were discharged within 4 to 8 days of their admission without major procedure-related complications.
TRICENTO transcatheter heart valve implantation with 3-dimensional MSCT-fluoroscopy fusion imaging guidance and follow-up CMR. Baseline right ventriculography showed severe tricuspid regurgitation (A). The 3-dimensional segmented model with landmarks was registered to fluoroscopy. Device implantation facilitated by MSCT-fluoroscopy fusion imaging (B, C). Final ventriculography demonstrated no systolic caval backflow, but dense opacification of the right atrium (D). Three and 6-month CMR showed significant reduction of the RV volumes and an improvement in RV function (E, F). CMR, cardiac magnetic resonance; EDV, end-diastolic volume; EF, ejection fraction; MSCT, multislice computed tomography; RV, right ventricle.
During follow-up (11±4.4 months), all patients showed NYHA functional class improvement (all of them in NYHA class I-II). No patients died during the follow-up. One patient was admitted with acute decompensation of HF (41 days after the procedure) and 1 patient required early postdischarge follow-up at HF clinic-day hospital for therapeutic optimization. The 6-month follow-up transthoracic echocardiogram showed a reduction in TR grade (4 of 6 patients showed a reduction in TR of ≥ 1 grade, P=.059), vena contracta width, tricuspid annular diameter, and RA volume. Right ventricular (RV) function, assessed by TAPSE, S’ and fractional area change, improved after the procedure, but this improvement was not statistically significant. The prostheses were correctly positioned, with no backflow in the venae cavae, no obstruction of the hepatic veins or leaks. All patients continued under oral anticoagulation with acenocumarol and diuretic doses were reduced during follow-up; even so, N-terminal pro-B-type natriuretic peptide levels were lower, although this decrease was not statistically significant ().
The main limitation of this study is its observational nature and the small number of patients included. Relevant follow-up data could not be obtained (functional and quality of life assessment, multimodality-imaging, and right heart catheterization at follow-up). This task must be addressed in further studies.
In summary, we report data from the first observational, prospective and multicenter study on the safety and feasibility of TTHV implantation for severe functional TR. TTHV may reflect a safe and easy alternative to improve functional class and symptoms related to HF for selected high-risk patients with severe TR. Furthermore, mid-term follow-up outcomes are promising, showing a positive RV reverse remodeling and improved RV functional parameters. During follow-up, the presence of the TTHV prevents backflow in the venae cavae and would provide a more directional flow toward the RA and improve cardiac output, which in the presence of preserved RV function and absence of pulmonary hypertension, would maintain the autoregulatory capacities of the RV. This is an important point because this device could theoretically lead to a chronic overload and progressive ventricularization of the RA, as well as to an overload and progressive remodeling of the RV. These hemodynamic concerns have not been observed. However, recently systolic compression of the stent at the level of the RA and functional ventricularization of the RA has been described by Willbring et al.5 Further studies are warranted to validate these initial results.