Timothy syndrome (TS) type 2 is an extremely rare, treatment-resistant condition. Our current knowledge of TS type 2 is based on a few case reports. Hermida et al.1 previously reported the case of a young patient with TS type 2 and a p. (Gly402Ser) mutation, who was followed up for 9 years at Amiens University Hospital. The authors found that a combination of mexiletine and nadolol was partially effective in reducing the delivery of appropriate shocks by the boy's defibrillator. The patient's favorable outcome after replacement of mexiletine by ranolazine prompted us to publish these new data. The patient's parents gave their written informed consent for publication of the present report.
In December 2010, a 32-month-old boy was resuscitated from a cardiac arrest at home. No structural heart disease was found, and the initially recorded electrocardiograms were suggestive of congenital long QT syndrome. In 2016, next-generation sequencing with a 51-gene panel revealed a heterozygous c.1204G > A; p.(Gly402Ser) mutation in exon 8 of the CACNA1C gene. We confirmed the presence of the mutation by analyzing a separate saliva sample. Neither of the parents carried the mutation in the tissues studied (blood and saliva), and none of the family members had similar cardiological features. Thus, we considered the mutation to be de novo.
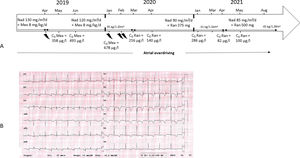
Following the implantation of a cardioverter defibrillator when the boy was aged 2.5 years, he received a total of 29 appropriate shocks over the following 10 years (figure 1A). The patient was treated alternately with nadolol alone (two 22-month periods) and in combination with mexiletine (an 11-month period and a 54-month period). We also decided to perform left cardiac sympathetic denervation, and initiated atrial overdrive pacing at 100/min.
A. Timeline for the 3 last years of follow-up. B. A typical electrocardiogram recorded while the patient was on nadolol plus ranolazine. CA, cardiac arrest; C0, residual concentration; Fleca, flecainide; ICD, implantable cardioverter defibrillator; LCSD, left cardiac sympathetic denervation; Mex, mexiletine; And, nadolol; Ran, ranolazine.
The thunder symbol represents ICD discharge.
The patient's status worsened in early 2020, with 3 appropriate shocks in 2 months while the patient was on nadolol plus mexiletine. In early March 2020, we replaced mexiletine with a sustained release (SR) formulation of ranolazine. We began with a dose of 375 mg SR twice a day; at that time, the patient weighed 35 kg. Treatment with this dose led to significant QT prolongation, with an increase in the mean ± standard deviation value from 563 ± 15 ms (the mean from 10 electrocardiograms [ECGs] recorded from January to February 2020) to 587 ± 15ms for (the mean from 16 ECGs recorded in March 2020). We decreased the dose to 375 mg of ranolazine SR once daily, and the QTc interval stabilized at a mean value of 568 ± 17 ms (for 26 ECGs recorded between March 2020 and April 2021; figure 1B). The patient's blood ranolazine concentration was assayed 3 times between the switch and April 2021 and was always in the expected therapeutic range (126-318 ng/mL). In late April 2021, his blood concentration fell below the therapeutic range, and so we increased the dose of ranolazine SR to 500 mg once daily.
The patient did not receive any appropriate shocks during the following 18 months on nadolol plus ranolazine; this contrasted with 4 appropriate shocks during the 12 months before the switch. It should be noted, on the one hand, that the patient had already been shock-free for the 17 months between November 2015 and May 2017, and therefore factors other than the treatment (eg, hormonal changes in preadolescence) might explain the current lull. On the other hand, the patient has not taken any concomitant medication and there have been no changes in blood potassium or physical activity levels; this rules out a number of confounding factors that might otherwise have accounted for the patient's current shock-free period.
The results of in vitro simulations suggest that ranolazine is effective in normalizing arrhythmia triggers in bradycardia-dependant arrhythmia and in type 3 long QT syndrome.2 In the study by Chorin et al.3 study of patients with type 3 long QT syndrome, treatment with ranolazine resulted in a shorter QT interval.
In vitro data supporting the efficacy of ranolazine in TS were reported in 2007.4 Clinical data are scarce: the only report on the use of ranolazine to treat an adult patient with TS type 2 and the p.(Gly402Ser) mutation was published by Shah et al.5 in 2012. Here, we report a second case of the successful treatment of TS type 2 with ranolazine, with an 18-month follow-up period. We consider that ranolazine was effective because it suppressed all episodes of ventricular arrhythmia, even though we did not observe QTc shortening; this was also true for the case reported by Shah et al. The effects of therapeutic concentrations of ranolazine on cardiac ion currents include inhibition of IKr, late INa and late ICa,L currents. Inhibition of IKr ranolazine prolongs the action potential duration (APD), whereas its inhibition of late INa and late ICa,L shortens the APD. The net clinical impact of the inhibition of these ion channel currents is a modest increase in the mean QTc interval, as observed in the present case. The ability of ranolazine to produce multi-current inhibition (and particularly its ability to potently block the late INa current) probably underlies its ability to prolong QT without creating the substrate or trigger for the development of Torsade de Pointe. Indeed, this feature could contribute to the suppression of early after-depolarizations and a reduction in the spatial dispersion of repolarization (the substrate and trigger for Torsade de Pointe) and so might account for ranolazine's significant antiarrhythmic activity. The suppression of early after-depolarizations also results from the ranolazine-induced reduction in [Ca2+]i, which is known to modulate triggered activity.
Since there are no guidelines on the optimal dose for ranolazine in children, we monitored the blood concentration. This therapeutic drug monitoring prompted us to administer the drug once a day (rather than twice a day, as in adults), with clinical effectiveness.
The present case report is the first to describe the successful treatment of pediatric TS type 2 with ranolazine. Use of the drug has suppressed the need for appropriate shocks for the last 18 months.
FUNDINGThis research did not receive any specific funding from agencies or organizations in the public, commercial, or not-for-profit sectors.
AUTHORS’ CONTRIBUTIONSConceptualization, formal analysis, writing, review and editing, visualization: A. Hermida, and J. S. Hermida; methodology, validation: A. Hermida, G. Jedraszak, M. Kubala, and J. S. Hermida; investigation, data curation: A. Hermida, G. Jedraszak, M. Kubala, M. Bourgain, S. Bodeau, and J. S. Hermida; resources: A. Hermida, M. Kubala, S. Bodeau, and J. S. Hermida; writing, original draft: A. Hermida; supervision: J. S. Hermida.
CONFLICTS OF INTERESTNone declared.
.