In recent years, several studies have shown a high incidence of adverse clinical events following acute coronary syndrome (ACS).1–4 Abu-Assi et al1 suggested using a new risk scoring system to predict post-discharge cardiovascular events in patients with ACS. However, as the authors acknowledge, one of the main limitations of their excellent work was the lack of an external validation process. Therefore, to improve the validity of this new scoring system, we assessed its predictive power and discriminatory power in a contemporary cohort of patients with ACS.
We carried out a retrospective study in accordance with the principles of the Declaration of Helsinki. The study was based on the data from a prospective registry of all patients with ACS admitted to a tertiary hospital in Spain. The inclusion period was from January 2012 to September 2014 (n=1039). The study excluded patients who died in hospital (n=55), those lost to follow-up (n=7), and those who had incomplete data to calculate the score (n=95; 98% due to unknown coronary anatomy). Thus, the final cohort comprised 882 patients. Follow-up at 1 year was carried out by a team of cardiologists and nursing staff, via telephone calls and review of clinical notes. Study events (cardiovascular death, acute myocardial infarction, and stroke) were defined according to the definitions used in the original study by Abu-Assi et al.1 Causes of death were determined from information obtained from telephone calls to patients’ relatives, review of clinical notes, and death certificates. In cases of uncertainty, or if the hospital notes were ambiguous or unavailable, we consulted the death register. Discriminatory power was analyzed by calculating the value of the area under the ROC curve. Calibration of the model was evaluated using the Hosmer-Lemeshow goodness of fit test. Calibration and discriminatory power were calculated for the total population and by subgroup according to type of ACS.
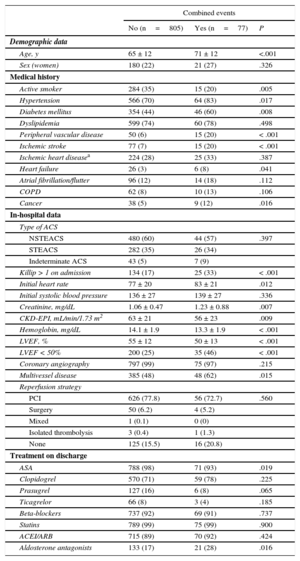
During the first year post-discharge, of 882 patients, 77 (8.7%) had a combined study event: 48 (5.4%) died of cardiovascular causes, 44 (5.0%) had a re-infarct, and 10 (1.1%) had a stroke. Table 1 compares patients with and without adverse clinical events: patients with adverse clinical events were older, had a higher rate of comorbidities (hypertension, diabetes mellitus, peripheral vascular disease, cerebrovascular disease, previous heart failure, and cancer) and had worse Killip class and renal function. They also had lower left ventricular ejection fraction and hemoglobin, and greater extent of coronary disease.
Baseline Population Characteristics According to Adverse Events at 1 Year
| Combined events | |||
|---|---|---|---|
| No (n=805) | Yes (n=77) | P | |
| Demographic data | |||
| Age, y | 65 ± 12 | 71 ± 12 | <.001 |
| Sex (women) | 180 (22) | 21 (27) | .326 |
| Medical history | |||
| Active smoker | 284 (35) | 15 (20) | .005 |
| Hypertension | 566 (70) | 64 (83) | .017 |
| Diabetes mellitus | 354 (44) | 46 (60) | .008 |
| Dyslipidemia | 599 (74) | 60 (78) | .498 |
| Peripheral vascular disease | 50 (6) | 15 (20) | < .001 |
| Ischemic stroke | 77 (7) | 15 (20) | < .001 |
| Ischemic heart diseasea | 224 (28) | 25 (33) | .387 |
| Heart failure | 26 (3) | 6 (8) | .041 |
| Atrial fibrillation/flutter | 96 (12) | 14 (18) | .112 |
| COPD | 62 (8) | 10 (13) | .106 |
| Cancer | 38 (5) | 9 (12) | .016 |
| In-hospital data | |||
| Type of ACS | |||
| NSTEACS | 480 (60) | 44 (57) | .397 |
| STEACS | 282 (35) | 26 (34) | |
| Indeterminate ACS | 43 (5) | 7 (9) | |
| Killip > 1 on admission | 134 (17) | 25 (33) | < .001 |
| Initial heart rate | 77 ± 20 | 83 ± 21 | .012 |
| Initial systolic blood pressure | 136 ± 27 | 139 ± 27 | .336 |
| Creatinine, mg/dL | 1.06 ± 0.47 | 1.23 ± 0.88 | .007 |
| CKD-EPI, mL/min/1.73 m2 | 63 ± 21 | 56 ± 23 | .009 |
| Hemoglobin, mg/dL | 14.1 ± 1.9 | 13.3 ± 1.9 | < .001 |
| LVEF, % | 55 ± 12 | 50 ± 13 | < .001 |
| LVEF < 50% | 200 (25) | 35 (46) | < .001 |
| Coronary angiography | 797 (99) | 75 (97) | .215 |
| Multivessel disease | 385 (48) | 48 (62) | .015 |
| Reperfusion strategy | |||
| PCI | 626 (77.8) | 56 (72.7) | .560 |
| Surgery | 50 (6.2) | 4 (5.2) | |
| Mixed | 1 (0.1) | 0 (0) | |
| Isolated thrombolysis | 3 (0.4) | 1 (1.3) | |
| None | 125 (15.5) | 16 (20.8) | |
| Treatment on discharge | |||
| ASA | 788 (98) | 71 (93) | .019 |
| Clopidogrel | 570 (71) | 59 (78) | .225 |
| Prasugrel | 127 (16) | 6 (8) | .065 |
| Ticagrelor | 66 (8) | 3 (4) | .185 |
| Beta-blockers | 737 (92) | 69 (91) | .737 |
| Statins | 789 (99) | 75 (99) | .900 |
| ACEI/ARB | 715 (89) | 70 (92) | .424 |
| Aldosterone antagonists | 133 (17) | 21 (28) | .016 |
ACEI, angiotensin-converting enzyme inhibitors; ACS, acute coronary syndrome; ARB, angiotensin receptor blockers; ASA, acetylsalicylic acid; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; NSTEACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEACS, ST-segment elevation acute coronary syndrome.
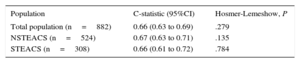
On the new scoring system, individuals with adverse events had a higher score than those without events: 8.01 ± 4.18 points versus 5.64 ± 4.51 points. Furthermore, analysis by category showed that as the risk score increased, the percentage of events also increased progressively (< 3 points, 3.3%; 3-7 points, 7.3%; and > 7 points, 13.5%; P<.001). The calibration of this scoring system was good, but the discriminatory analyses showed an acceptable (not excellent) predictive power for cardiovascular events during the first year post-discharge (C-statistics<0.7) (Table 2).
Discrimination and Calibration of Scoring in the Total Population and in the Different Subgroups
| Population | C-statistic (95%CI) | Hosmer-Lemeshow, P |
|---|---|---|
| Total population (n=882) | 0.66 (0.63 to 0.69) | .279 |
| NSTEACS (n=524) | 0.67 (0.63 to 0.71) | .135 |
| STEACS (n=308) | 0.66 (0.61 to 0.72) | .784 |
95%CI, 95% confidence interval; NSTEACS, non-ST segment elevation acute coronary syndrome; STEACS, ST-segment elevation acute coronary syndrome.
In this study, we validated for the first time a recently proposed new scoring system for prediction of cardiovascular events during the first year post-discharge from hospital in patients with ACS. In our study, this new scoring system had an acceptable discriminatory power and calibration, in both the total population and in the 2 subgroups analyzed, which indicates its potential clinical usefulness as a stratification tool for patients with ACS in our environment.