Two-dimensional speckle-tracking echocardiography has emerged as a promising alternative to endomyocardial biopsy to rule out acute cellular rejection after orthotopic heart transplantation (OHT) in single center studies. In an original cohort, 15.5% and 17% of cutoff points for left ventricular global longitudinal strain (LVGLS) and free-wall right ventricular longitudinal strain, respectively, achieved 100% negative predictive value to exclude moderate or severe acute cellular rejection (ACR ≥ 2R). Our objective was to demonstrate the usefulness of speckle-tracking and validate these cutoff points in an external cohort.
MethodsA prospective, multicenter study that included patients who were monitored during their first year after OHT was conducted. Echocardiographic studies analyzed by local investigators were compared with simultaneous paired endomyocardial biopsies samples.
ResultsA total of 501 endomyocardial biopsy-echocardiographic studies were included in 99 patients. ACR≥2R was present in 7.4% of samples. LVGLS and free-wall right ventricular longitudinal strain were significantly reduced during ACR≥2R on univariate analysis. On multivariate analysis, LVGLS was independently associated with the presence of ACR≥2R. The original cutoff points demonstrated a negative predictive value of 94.3% to exclude ACR≥2R.
ConclusionsThis study maintained a strong negative predictive value to exclude ACR≥2R after OHT and LVGLS was independently associated with the presence of ACR≥2R. We propose the use of speckle-tracking, especially LVGLS, as part of the noninvasive diagnosis and management of ACR.
Keywords
Advances in immunosuppression have led to a decrease in the incidence of acute cellular rejection (ACR) after orthotopic heart transplant (OHT).1 However, ACR is still a major concern as its presence is related to graft loss and reduced long-term survival.2 Thus, active ACR surveillance after OHT is mandatory. This is especially relevant given that the current “gold standard” technique for ACR detection is endomyocardial biopsy (EMB), which is an invasive method that is not free of complications.3,4 Echocardiography is a widely available technique and many investigations have evaluated its use in ACR diagnosis. Classic parameters have shown inconsistent results, with no single parameter capable of correctly diagnosing ACR.5–10
More recently, myocardial strain has emerged as a promising tool due to its higher sensitivity to detect myocardial dysfunction in many different scenarios.11,12 Several studies have reported a significant relationship between ACR and 2-dimensional speckle-tracking echocardiography (STE).13–15 In 2015, a study was published on the usefulness of left ventricular global longitudinal strain (LVGLS) and free-wall right ventricular longitudinal strain (RVLS) to exclude ACR in an single center cohort.16 Cutoff points of 15.5% and 17% for LVGLS and free-wall RVLS, respectively (absolute values), provided high sensitivity and specificity to exclude ACR and also achieved a negative predictive value (NPV) of 100% when both variables were combined.
These are single center studies, with different inclusion criteria, and propose different cutoff points for ACR diagnosis. Furthermore, not all studies have reported positive results.17,18 Our objective was to perform an external validation of the usefulness of STE and the original cutoff points to safely exclude ACR≥2R. We hypothesized that more sensitive measurements such as LVGLS and free-wall RVLS could be a useful and reproducible tool in the noninvasive management of ACR.
METHODSThis multicenter study was performed by 7 Spanish cardiac transplant centers. Patients admitted for OHT were consecutively and prospectively included in the study from December 2015 to December 2016 and were monitored during their first year after OHT. We evaluated pairs of EMB and echocardiographic studies performed within 24hours of the EMB and always before ACR treatment in patients requiring this treatment.
EMBs were performed periodically at 15 days, 1 month, 2 months, 3 months, 6 months and 1 year after OHT (). EMBs after a moderate or severe ACR were also included. EMBs were read by local pathologists and grading evaluation was established according to the 2005 International Society of Heart and Lung transplantation recommendations.19 These results were considered as the “gold standard” and the need for treatment in stable patients (generally accepted in cases with ACR≥2R) was left at the discretion of local clinicians. EMBs were routinely examined for histologic signs of antibody-mediated rejection. Immunopathologic techniques and assessment of circulating antihuman leukocyte antigen antibodies were performed according to local protocols. Echocardiography studies were performed and later analyzed internally by dedicated echocardiographers working in each center. Echocardiographers were blinded to EMB results. The main exclusion criteria were severe right or left primary graft failure according to the International Society for Heart and Lung Transplantation guidelines20 and the absence of an adequate echocardiographic window to evaluate STE parameters. Our study was performed in compliance with the Declaration of Helsinki and was approved by all local ethics committees. Written informed consent was provided by all study participants.
Data regarding patients’ demographic and clinical data and EMB results were sent and collected in a database. Each center maintained its own immunosuppressive protocol and post-OHT follow-up, including unscheduled visits if needed. Each center followed its own angiographic cardiac allograft vasculopathy (CAV) surveillance protocols.
Two-dimensional echocardiographyChamber size and classic assessment of cardiac functionAll studies were performed using echocardiographic equipment (IE33) from Phillips Medical Systems (Best, Netherlands). Cine loops from standard apical and parasternal views were recorded using grayscale harmonic imaging. Interventricular septum and posterior wall thickness, as well as end-systolic and end-diastolic diameters, were obtained from M-mode or 2-dimensional imaging in the parasternal long-axis view. LV and RV dimensions, left ventricular ejection fraction by the Simpson method, tricuspid annular plane systolic excursion and right ventricular fractional area change were calculated according to the American Society of Echocardiography recommendations.21 Mitral inflow was obtained by the calculation of pulsed-wave Doppler echocardiography and early (E) and late (A) ventricular filling velocities, (E/A) ratio, deceleration time, and isovolumetric relaxation time. Tissue Doppler imaging data from the septal and lateral mitral annulus were collected and the medial and lateral mitral E/E’ ratio were also measured. Tissue Doppler imaging was also used to calculate tricuspid peak systolic (Ś) velocity.
Speckle derived parametersPrior to the initiation of the study, echocardiographers from the different hospitals were brought together to standardize the criteria for STE-offline analysis. Each echocardiographer selected for this study had previous experience in STE analysis in their daily clinical practice. Echocardiographic studies were performed and analyzed in each hospital and then sent to an external echocardiographer of the organizing hospital who supervised the tracking and collected the data. When STE tracking was considered inadequate, local echocardiographers made new attempts to achieve a proper tracking. In the infrequent cases in which, despite several attempts by local echocardiographers, the tracking was considered inadequate by the central echocardiographer, the corresponding views were excluded and not included in the analysis.
Three consecutive cardiac cycles were digitally stored as raw data for subsequent offline analysis using commercial software (QLab version 10.2, 10.3 and 10.5) and the frame rate was optimized for each view (between 55 and 90 frames/sec). LVGLS was calculated as the average of the peak systolic strain obtained in apical 4-chamber view and 2-chamber view using a 12-segment model (the same model as that employed in the original cohort).16 Right ventricular global longitudinal strain was obtained using a 6-segment model and free-wall RVLS was measured as the average of the 3 lateral segments. Three regions of interest were selected in each view and strain values were automatically generated. Strain values are expressed in absolute numbers for the sake of clarity. Segments that failed to track properly were manually adjusted until correct tracking frame-by-frame was obtained. Views with more than 2 segments with inadequate endocardial visualization or tracking were excluded.
Statistical analysisThe normality of data distribution was evaluated using graphical methods and the Kolmogorov-Smirnov test. Continuous variables are expressed as mean± SD (or medians and interquartile ranges for variables not normally distributed) and categorical data as frequencies and percentages. For ACR excluding purposes, studies were divided into 2 groups according to the presence of ACR≥2R. The chi-square test and Student t-test were used for comparison of categorical and quantitative variables, respectively. For nonnormally distributed variables the Mann-Whitney U-test was used. A P value<.05 was considered to indicate statistical significance. Predictors of ACR≥2R selected on the basis of a P value <.05 were entered in a multivariate analysis. Binary logistic regression with a forward stepwise approach was used for the multivariate analysis. ROC curves for LVGLS and free-wall RVLS were calculated. Interobserver reproducibility was evaluated with the intraclass correlation coefficient and Bland-Altman plots. All analyses were carried out using SPSS version 20 (SPSS, Inc, Chicago, United States). Bland-Altman plots were performed using R (R Core Team, 2019).22
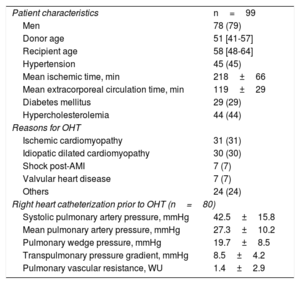
RESULTSFrom December 2015 to December 2016, 99 patients were included in the study (). Five patients were excluded due to a suboptimal echocardiographic window. We initially included 516 EMB and echocardiographic paired studies, although 15 (2.9%) pairs were excluded due to insufficient material in EMB that precluded their comparison with echocardiograms. Finally, 501 EMB and their corresponding echocardiographic evaluations were analyzed. The average number of EMB per patients was 4 (range: 1-10). The patients’ baseline characteristics of patients are shown in table 1.
Patient characteristics (n=99)
| Patient characteristics | n=99 |
| Men | 78 (79) |
| Donor age | 51 [41-57] |
| Recipient age | 58 [48-64] |
| Hypertension | 45 (45) |
| Mean ischemic time, min | 218±66 |
| Mean extracorporeal circulation time, min | 119±29 |
| Diabetes mellitus | 29 (29) |
| Hypercholesterolemia | 44 (44) |
| Reasons for OHT | |
| Ischemic cardiomyopathy | 31 (31) |
| Idiopatic dilated cardiomyopathy | 30 (30) |
| Shock post-AMI | 7 (7) |
| Valvular heart disease | 7 (7) |
| Others | 24 (24) |
| Right heart catheterization prior to OHT (n=80) | |
| Systolic pulmonary artery pressure, mmHg | 42.5±15.8 |
| Mean pulmonary artery pressure, mmHg | 27.3±10.2 |
| Pulmonary wedge pressure, mmHg | 19.7±8.5 |
| Transpulmonary pressure gradient, mmHg | 8.5±4.2 |
| Pulmonary vascular resistance, WU | 1.4±2.9 |
AMI, acute myocardial infarction; OHT, orthotopic heart transplantation.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
Table 2 shows the ACR degrees found during follow-up in the 501 EMB. We divided EMB into 2 groups according to the presence of ACR≥2R. ACR≥2R was present in 37 samples (7.4%) and corresponded to 26 patients, with 9 patients having more than one ACR≥2R episode. Immunopathologic signs of antibody-mediated rejection were present in 3 studies (pAMR1-I) with no other rejection signs or symptoms. None of them were considered significant and none of them had ACR≥2R. There was 1 death due to resistant ACR at the time of the third EMB. The remaining deaths during follow-up were due to 2 cases of sepsis, one sudden cardiac death (in a patient without previous coronary angiography) and 1 case of multiorgan failure (no echocardiographic studies with STE analysis due to the patients's indolent clinical course).
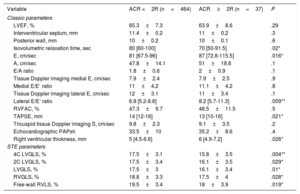
Table 3 shows conventional and STE echocardiographic parameters related to the presence of ACR≥2R in the univariate analysis. Left ventricular ejection fraction was not significantly different between these 2 groups, nor was septal or posterior wall LV thickness. Isovolumetric relaxation time was significantly shorter in patients with ACR≥2R. In addition, E and lateral E/E’ ratios were notably higher. Significant pericardial effusion (moderate or severe) was more frequent in ACR≥2R studies (8.3% vs 24.3%, P=.008).
Conventional, Doppler-derived and STE echocardiographic parameters and relationship with the presence of ACR≥2R on univariate analysis (STE results are presented in absolute values)
| Variable | ACR <2R (n=464) | ACR≥2R (n=37) | P |
|---|---|---|---|
| Classic parameters | |||
| LVEF, % | 65.3±7.3 | 63.9±8.6 | .29 |
| Interventricular septum, mm | 11.4±0.2 | 11±0.2 | .3 |
| Posterior wall, mm | 10±0.2 | 10±0.1 | .6 |
| Isovolumetric relaxation time, sec | 80 [60-100] | 70 [50-91.5] | .02* |
| E, cm/sec | 81 [67.5-96] | 87 [72.8-115.5] | .016* |
| A, cm/sec | 47.8±14.1 | 51±18.8 | .1 |
| E/A ratio | 1.8±0.6 | 2±0.9 | .1 |
| Tissue Doppler imaging medial E, cm/sec | 7.9±2.4 | 7.9±2.5 | .9 |
| Medial E/E’ ratio | 11±4.2 | 11.1±4.2 | .8 |
| Tissue Doppler imaging lateral E, cm/sec | 12±3.1 | 11±3.4 | .1 |
| Lateral E/E’ ratio | 6.8 [5.2-8.8] | 8.2 [5.7-11.3] | .009** |
| RVFAC, % | 47.3±9.7 | 48.5±11.5 | .5 |
| TAPSE, mm | 14 [12-16] | 13 [10-16] | .021* |
| Tricuspid tissue Doppler imaging Ś, cm/sec | 9.8±2.3 | 9.1±3.5 | .2 |
| Echocardiographic PAPsh | 33.5±10 | 35.2±8.6 | .4 |
| Right ventricular thickness, mm | 5 [4.5-6.6] | 6 [4.9-7.2] | .026* |
| STE parameters | |||
| 4C LVGLS, % | 17.5±3.1 | 15.8±3.5 | .004** |
| 2C LVGLS, % | 17.5±3.4 | 16.1±3.5 | .029* |
| LVGLS, % | 17.5±3 | 16.1±3.4 | .01* |
| RVGLS, % | 18.8±3.3 | 17.5±4 | .028* |
| Free-wall RVLS, % | 19.5±3.4 | 18±3.9 | .019* |
2C LVGLS, 2-chamber view left ventricular longitudinal strain; 4C LVGLS, 4-chamber view left ventricular longitudinal strain; ACR, acute cellular rejection; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain; PAPs, systolic pulmonary artery pressure; RVFAC, right ventricular fractional area change; RVGLS, right ventricular global longitudinal strain; RVLS, right ventricular longitudinal strain; STE, speckle-tracking echocardiography; TAPSE, tricuspid annular plane systolic excursion.
The data are expressed as mean±standard deviation or median [interquartile range].
LVGLS and right ventricular global longitudinal strain were significantly reduced in patients with ACR≥2R in the univariate analysis (figure 1). LVGLS was 17.5±3% in patients with ACR <2R and 16.1%±3.4 in ACR≥2R (P=.01). Similar differences between the 2 groups were observed in free-wall RVLS (19.5%±3.4 vs 18%±3.9 for ACR <2R and ACR≥2R respectively, P=.019).
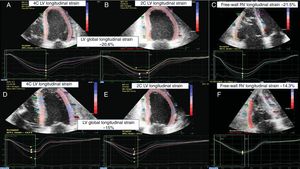
Echocardiographic STE imaging of a patient. A, B and C: EMB graded as 0R. D, E and F: an ACR=2R episode. From left to right: 4-chamber LV longitudinal strain, 2-chamber LV longitudinal strain, and free-wall RV longitudinal strain. ACR, acute cellular rejection; EMB, emdomyocardial biopsy; LV, left ventricular; RV, right ventricular; STE, 2-dimensional speckle-tracking echocardiography.
In the multivariate analysis, isovolumetric relaxation time, lateral E/E’ ratio and LVGLS remained independently related to the absence of ACR≥2R (table 4). LVGLS was the best parameter with regard to the absence of ACR≥2R.
Echocardiographic parameters related to the absence of ACR≥2R on multivariate analysis
| Variable | OR (95%CI) | P |
|---|---|---|
| LVGLS, % | 1.23 (1.1-1.4) | .01 |
| IVRT | 1.01 (1-1.03) | .04 |
| Lateral E/E’ ratio | 0.9 (0.82-0.98) | .02 |
95%CI, 95% confidence interval; IVRT, isovolumetric relaxation time; LVGLS, left ventricular global longitudinal strain; OR, odds ratio.
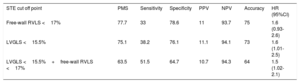
The area under the receiver operating characteristic curve was 0.67 for LVGLS and 0.60 for free-wall RVLS. LVGLS and free-wall RVLS original cutoff points (absolute values being 15.5% and 17%, respectively), as well as categorical variables corresponding to patients with LVGLS >15.5% and free-wall RVLS> 17% (LVGLS> 15.5%+free-wall RVLS> 17%), were applied to our cohort. Table 5 reflects test performance results for each value to exclude ACR≥2R. NPV was 93.7% for free-wall right ventricular global longitudinal strain, 94.1% for LVGLS, and 94.3% for LVGLS+free-wall RVLS.
LVGLS and free-wall RVLS original cutoff points related to the diagnosis of ACR≥2R and their prevalence, sensitivity, specificity, positive predictive value, negative predictive value, hazard ratios, and accuracy
| STE cut off point | PMS | Sensitivity | Specificity | PPV | NPV | Accuracy | HR (95%CI) |
|---|---|---|---|---|---|---|---|
| Free-wall RVLS <17% | 77.7 | 33 | 78.6 | 11 | 93.7 | 75 | 1.6 (0.93-2.6) |
| LVGLS <15.5% | 75.1 | 38.2 | 76.1 | 11.1 | 94.1 | 73 | 1.6 (1.01-2.5) |
| LVGLS <15.5%+free-wall RVLS <17% | 63.5 | 51.5 | 64.7 | 10.7 | 94.3 | 64 | 1.5 (1.02-2.1) |
95%CI, 95% confidence interval; ACR, acute cellular rejection; HR, hazard ratio; LVGLS, left ventricular global longitudinal strain; NVP, negative predictive value; PMS, prevalence of the measures in the sample; PPV, positive predictive value; RVLS, right ventricular longitudinal strain; STE, speckle-tracking ecochardiography.
Hazard ratios for univariate analysis predicting ACR≥2R.
Unless otherwise indicated, data are presented as percentages.
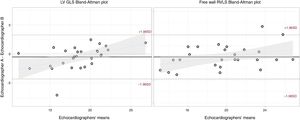
Among the 501 EMB and echocardiographic studies initially included, STE analysis was not possible due to technical reasons in 20 studies (4%). Among the remaining 481 studies, 2.3% for LVGLS and 6.6% for free-wall RVLS could not be analyzed due to inadequate endocardial visualization despite manual adjustment. Interobserver reproducibility was assessed in 28 studies, including echocardiograms from all the centers. The global intraclass correlation coefficient for LVGLS was 0.86 (95%CI, 0.72-0.93) and 0.93 (95%CI, 0.86-0.97) for free-wall RVLS. Bland-Altman plots are shown in figure 2.
Bland-Altman analysis for interobserver variability. Bland-Altman analysis for left ventricular global longitudinal strain (left) and free-wall right ventricular longitudinal strain (right). LVGLS, left ventricular global longitudinal strain; RVLS, right ventricular longitudinal strain.
CAV was classified angiographically according to the ISHLT classification (CAV0-3).23 Two patients had severe CAV (CAV3). The first patient had an initial angiogram with no significant stenosis and several months after OHT had an acute myocardial infarction. Only echocardiographic studies previous to the infarction were included. The second patient had no episodes of ACR≥2R during follow-up, nor did the only patient who had moderate CAV (CAV2). Coronary angiography status 1 year after OHT was unknown in 26 patients (26.2%). In most cases, this was due to local angiographic CAV surveillance protocols that performed the first surveillance angiogram later than 1 year after OHT.
DISCUSSIONIn this multicenter study, we sought to validate the value of STE parameters to exclude ACR in asymptomatic patients during the first year after OHT. To the best of our knowledge, this is the first multicenter study to evaluate the usefulness of STE in ACR after OHT. We also assessed the usefulness of RV strain analysis, which has been poorly described to date in this scenario.
Our study confirms the reduction in left ventricular and right ventricular STE parameters during ACR≥2R. Furthermore, in the multivariate analysis, LVGLS was independently associated with the presence of ACR≥2R. Finally, in this study, we demonstrate a strong NPV when both LVGLS and free-wall RVLS are combined, providing at first glance a helpful tool in the noninvasive management of ACR and in the optimization of immunosuppression regimes.
STE parameters have been previously proposed in several studies as a tool to noninvasively diagnose ACR, but this is still a matter of debate.13–18 Previous studies had heterogeneous populations and inclusion criteria, were performed in single centers, and not all demonstrated a significant reduction in LVGLS or any other strain parameters in ACR scenarios.17,18 In this regard, most of the studies describe the usefulness of LVGLS later after OHT and the evidence supporting its use during the first year after OHT is smaller.13,16,18 Ambardekar et al.18 failed to demonstrate changes in STE analysis during asymptomatic rejection episodes (any degree of ACR). Our study only considered ACR≥2R, as asymptomatic mild ACR (ACR=1R) are not generally treated, which may partially explain the difference in our findings.
A major strength of this study is its multicenter nature and a design conceived to be broadly applicable in OHT patients and to reflect real-world practice in heart transplant centers. This naturally led to higher variability and less favourable results than in the original cohort. Indeed, STE values showed higher overlap between the 2 groups (ACR <2R vs ACR≥2R) and there was a slight fall in NPV (NPV 94.3% in the present cohort vs 100% in the original cohort for the variable LVGLS> 15.5%+free-wall RVLS> 17%).16
After multivariate analysis, free-wall RVLS was not significantly associated with ACR≥2R occurrence. This results could have been influenced by several factors. First, the acquisition and analysis of right ventricular views is more challenging than those of LV apical views and therefore it may be less reliable than LVGLS in everyday practice. This is reinforced by the fact that more right ventricular segments (6.6%) were not analyzable compared with LVGLS (2.3%). In addition, the presence of pulmonary hypertension in patients with advanced heart failure is related to RV failure after OHT. Furthermore, early after many cardiac surgeries (not exclusively OHT) there is a natural change in contractility pattern and geometry with a relative loss of longitudinal shortening.24 All these facts contribute to right ventricular function after OHT even in the absence of ACR.
STE variability and reproducibility remain a major concern, not only among different vendors but also with strain software updates.25–28 There is general optimism that this limitation will decrease along with technological advances.12,29–31 Nowadays, it is recommended to perform serial echocardiographic studies with the same vendor and software, although the use of software updates seems unavoidable and performing studies with archaic technology has little relevance and use. The original cohort was analyzed with QLab 7 software. The cohort of this multicenter study was evaluated with its update, the QLab 10 software, which may partially explain the loss of ability to exclude ACR in this cohort using our original cutoff points.
EMB has been considered the “gold standard” for ACR diagnosis and it is generally accepted that ACR≥2R must be treated. However, the ability of EMB to be a true gold standard is questionable and the consideration of ACR≥2R should no longer be the only criteria for an adequate immunosuppressive regime.32,33 It is important to highlight the interobserver variability of the EMB, clearly observed by the CARGO and CARGO II investigators.34–37 This variability among pathologists is higher in samples with some degree of rejection. Since the agreement is higher on exclusion of ACR, the possibility of sampling error should be considered, as the EMB may not be taken from a rejection focus due to the patchy nature of ACR. Thus, EMBs may be incorrectly graded as absence of ACR and not be treated.
Gene expression profiling in peripheral blood has proved to be useful in ruling out ACR≥2R in low-risk patients and in developing a gene expression profiling-based strategy in order to rule out potential risks.33,36,37 It is currently the most commonly used method in the noninvasive management of ACR after OHT. However, the usefulness of gene expression profiling early after OHT (< 2 months) or after a recent ACR has not been tested.33,37 In CARGO II, an excellent NPV was maintained (98.1% for a 34 cutoff point) and it increased to 100% by modifying the cutoff point and simultaneously decreasing the positive predictive value to 2%. Similarly, we obtained an NPV of 94.3% for LVGLS+free-wall RVLS (with a positive predictive value of 10.7%). The prevalence of ACR≥2R in CARGO II was only 3.2% (vs 7.4% in our series), which contributes to the high NPV and the limited positive predictive value, a common finding in noninvasive methods to exclude ACR. Donor-derived cell-free DNA also enables an early noninvasive ACR diagnosis.38–40 However, initial methods required both recipient and donor genotypes limiting its application.38,39,41 Newer techniques seem promising in this field although further studies are needed to validate them.40,42
Compared with these alternatives, echocardiography provides information about other basic parameters beyond the presence of ACR that have prognostic and management implications (such as graft function) and that can be affected by other OHT-derived complications. In addition, these methods, as well as other imaging techniques such as magnetic resonance imaging, are not as accessible and affordable as echocardiography is. We believe STE parameters (particularly LVGLS according to our results) may be a useful tool as part of the noninvasive diagnosis and management of ACR. It may be especially useful in asymptomatic patients with no other signs or symptoms of rejection and higher STE absolute values (where higher NPV is expected). These patients could be considered as “low-risk” patients suitable for a noninvasive management of ACR with close follow-up, particularly in heart transplant centers where EMB are performed very close in time or in patients with EMB complications or difficulties. In addition, when performed in everyday clinical practice, its variation over time may also be of interest. A sharp decrease in STE parameters in absolute values (with lower NPV) compared with previous echocardiographic studies could also be helpful for clinicians as part of the management of OHT patients. Thus, we recommend its measurement in everyday clinical practice to improve the evaluation of these patients. In this study, we did not intend to replace EMB with STE analysis but to enrich the possibilities of noninvasive ACR management.
LimitationsThere are several limitations to this study. First, the low prevalence of ACR≥2R observed in our study may have overestimated the NPV. The need for continuous standardization of the STE technique should also be considered, so that no changes in software or vendors are a limitation, as may have been the case in our study. Equally, there was no central core laboratory for both biopsies and echo measurements although a second echocardiographer supervised all data. Despite our results reflecting a wider variability, our aim was to evaluate the effectiveness of STE in real-world practice. Finally, there was no common protocol for antibody-mediated rejection or CAV among centers, which may have slightly altered our results.
CONCLUSIONSOur results demonstrate a strong NPV when both LVGLS and free-wall RVLS are combined providing a feasible and helpful tool in the noninvasive management of ACR. Besides, LVGLS was independently associated with the presence of ACR≥2R. We propose the use of STE parameters particularly in clinically stable low-risk patients with higher STE absolute values in order to alleviate the burden of repeated EMB. Further studies with larger sample size are needed to confirm these results.
- -
STE parameters have been proposed in single center studies as a noninvasive alternative to EMB in ACR diagnosis.
- -
In an original cohort, cut-off points of 15.5% for LVGLS and 17% for free-wall RVLS demonstrated 100% NPV to exclude ACR.
- -
This is the first multicenter study to analyze the usefulness of STE parameters in ACR.
- -
Patients with ACR≥2R showed a reduction in LVGLS and free-wall RVLS on univariate analysis.
- -
LVGLS remained an independent predictor of ACR≥2R on multivariate analysis and was the best parameter related to the presence of ACR≥2R.
- -
LVGLS> 17% and free-wall RVLS> 15.5% showed an NPV of 94.3% to exclude ACR≥2R.
- -
STE parameters may be used as part of the in the noninvasive management of ACR.
This study received a research grant from the Working Group on Heart Failure and Heart Transplantation of the Spanish Society of Cardiology.
CONFLICTS OF INTERESTC.-H. Li reports speaker fees from Philips, outside the submitted work. The other authors declare no conflicts of interest.
The authors are grateful to Fina Casals, Zulaika Grille, Paula Blanco and all the Heart Transplantation and Cardiac Imaging Units that collaborated in this project.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.01.012