In cardiovascular (CV) prevention, interest in diet is focused on the Mediterranean diet because a great deal of high-quality scientific evidence, such as that of the PREDIMED study,1 has shown a 30% reduction in severe CV complications and overall mortality in individuals at high CV risk.
These results are not solely due to the beneficial effect of this diet on classical risk factors (lipid profile, hypertension, waist circumference, obesity, insulin resistance, type 2 diabetes mellitus [DM2], and carotid atherosclerosis),2 but to additional emerging risk factors (PREDIMED substudies). Antioxidants, anti-inflammatory molecules, and poyphenols3 improve endothelial function, increase nitric oxide synthesis, and reduce thrombosis. The concept of nutrigenomic modulation,4 implicated in the oxidation of low-density lipoprotein and in postprandial triglyceridemia, fundamentally involves epigenetics and establishes different intermediate and final dyslipidemia phenotypes.5 Although the Mediterranean diet is a sustainable paradigm of CV prevention,6 and despite the proximity of this diet, current data show that 60% of the Spanish population with high cardiovascular risk is obese. In advanced stages, obesity reduces life expectancy by 10 years due to CV death, overall mortality, and cancer.7
PHYSICAL EXERCISEThe importance of regular physical exercise is more and more convincing, not only to prevent ischemic events in individuals with CV risk factors, but also to prevent the development of heart failure.8 However, recent studies are now answering the question of whether there is a J curve for physical exercise. A recently published study9 involving more than 1 million women and a 9-year follow-up found that 49 113 women had a primary coronary event, 17 822 had a first cerebrovascular event, and 14 550 had a first venous thromboembolic event. Women reporting moderate activity had a significantly lower risk of all 3 events than inactive women (all P < .001). Conversely, women who engaged in strenuous physical activity had a higher risk of coronary events (P = .002), cerebrovascular disease (P < .001), and venous thromboembolic events (P < .001) than moderately active women. Thus, it appears that there is indeed a J curve for physical activity.
Finally, an increasing number of initiatives promote early “active and heart-healthy lifestyles”, even in school-going children.10
SMOKINGSmoking remains the leading cause of preventable death and our main public health problem. According to Eurobarometer 2015 data, the prevalence of smoking in Spain is 29% (35% in men and 25% in women), slightly higher than the European average of 26%.
The latest revision of “Smoking and Health: Report of the Advisory Committee of the Surgeon General of the Public Health Service” was published in 2014; the report summarizes new evidence on the impact of smoking on age-related macular degeneration, DM2 (30%-40% higher risk for smokers), colorectal and liver cancer, tuberculosis, erectile dysfunction, orofacial clefts in the offspring of smokers, ectopic pregnancy, rheumatoid arthritis, inflammation, and impaired immune function. In addition, exposure to passive smoking is associated with a 20% to 30% increased risk of stroke, as well as higher risk of acute myocardial infarction, lung cancer, acute respiratory diseases, and sudden infant death syndrome.11
In a large prospective series evaluating the effects of smoking and smoking cessation in a cohort of more than 200 000 American adults,12 all-cause death was 3 times greater in smokers than in never smokers, with a life expectancy reduction of more than 10 years, largely due to neoplasms and respiratory and CV diseases. Smoking cessation before 40 years of age reduced the risk of smoking-related death by 90%.
One factor that has triggered wide social debate, and some differences of opinion among health care professionals, is the electronic cigarette. Those who defend its benefits as a preventive or risk reduction strategy have challenged the precautionary principle espoused by the World Health Organization, the main scientific societies, including the Spanish Society for Cardiology, and the Spanish National Committee of Smoking Prevention. There is still no solid evidence indicating that the electronic cigarette is an effective strategy to stop smoking,13 there are persistent doubts about its long-term safety, and, above all, from the point of view of public health, there are concerns that the electronic cigarette could become a gateway to nicotine addiction. Thus, and given the existence of safe and effective alternative strategies, the electronic cigarette should not be recommended to patients wishing to quit smoking.14
A systematic review investigating the impact of smoking on the CV morbidity and mortality of diabetic patients found a 50% higher CV mortality rate in diabetic patients who smoked, with a significant reduction in those who managed to quit.15
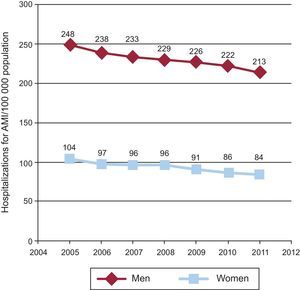
The Report to the Spanish Parliament evaluating the impact on public health of Law 42/2010, which extended the Spanish smoking ban to public places, summarized the benefits of this law on public health: a reduced prevalence of daily smokers (from 26.2% in 2009 to 24.0% in 2011), a 90% reduction in pollution from nicotine and fine particles in recreational areas, and fewer admissions for acute myocardial infarction, ischemic heart disease, and asthma. In the case of acute myocardial infarction in people older than 24 years, 2 falls in the admission rates were seen after the introduction of the law (2006) and its amendment (2011), of about 4% for each law in men, and a notable general reduction between 2005 and 2011 in both sexes (Figure 1).
Distribution of the rate of hospitalization for acute myocardial infarction per 100 000 population adjusted by sex and age. AMI, acute myocardial infarction. Data extracted from the Spanish Ministry of Health, Social Services, and Equality. Available at: http://www.msssi.gob.es/estadEstudios/estadisticas/cmbdhome.htm
A recently published updated treatment protocol for patients with coronary heart disease recommended that clinicians include the correct management of other risk factors when treating hypertension, especially in patients with heart failure, individualize the main antihypertensive treatment according to the concomitant disease, and maintain the therapeutic targets of systolic blood pressure below 140/90mmHg. In some selected patients, values less than 130/80mmHg can be recommended, but always in conjunction with a slow reduction in the arterial pressure values with medical therapy. For octogenarians with orthostatic hypotension, such low targets are not required.16
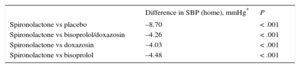
The new clinical trials presented at the 2015 European Congress of Cardiology have shown the superior efficacy of spironolactone over doxazosin and bisoprolol in combating resistant hypertension, defined as uncontrolled hypertension despite treatment with the maximum tolerated doses of 3 antihypertensive medications (angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, calcium antagonists, and thiazides) in the PATHWAY-2 trial17 (Table 1). The PATHWAY-3 trial18 showed that a combination of 2 diuretics, amiloride and hydrochlorothiazide, significantly reduced blood pressure to a greater extent than each drug alone and had a beneficial effect on glucose metabolism and potassium concentration. Thus, according to the current evidence, the effective treatment of hypertension could actually be quite inexpensive.
Primary Endpoint of the PATHWAY-2 Study
| Difference in SBP (home), mmHg* | P | |
|---|---|---|
| Spironolactone vs placebo | –8.70 | < .001 |
| Spironolactone vs bisoprolol/doxazosin | –4.26 | < .001 |
| Spironolactone vs doxazosin | –4.03 | < .001 |
| Spironolactone vs bisoprolol | –4.48 | < .001 |
SBP, systolic blood pressure.
Difference in systolic blood pressure (home readings) and comparators (n = 314).
Data extracted from Williams et al.17
In the field of lipids, notable recent publications include the results of the IMPROVE-IT study19 and the new data obtained from the ODYSSEY LONG TERM20 and OSLER21 trials on the efficacy, safety, and CV events of PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors.
The IMPROVE-IT trial evaluated the usefulness of ezetimibe in patients treated with statins after acute coronary syndrome (ACS). In that study, 18 144 patients were randomized to simvastatin in monotherapy or simvastatin and ezetimibe together, with a mean follow-up of 6 years. The primary endpoint was a composite of CV death, nonfatal myocardial infarction, unstable angina with rehospitalization, coronary revascularization (at least 30 days after randomization), and nonfatal stroke. During hospitalization due to ACS, the mean low-density lipoprotein cholesterol (LDL-C) was 93.8mg/dL; at 1 year, it was 69.9mg/dL in the simvastatin monotherapy group and 53.2mg/dL in the simvastatin-ezetimibe group (P < .001), with a 24% reduction in the LDL-C values with the simvastatin-ezetimibe combination. Regarding efficacy (Table 2), the incidence of the primary endpoint at 7 years was 32.7% in the simvastatin-ezetimibe group and 34.7% in the simvastatin monotherapy group (absolute risk reduction, 2.0%; P = .016). There were no significant differences in CV or all-cause death. There were also no differences between the groups in safety data, namely, hepatic enzyme elevation or adverse muscular effects. The hazard ratio (HR) for the clinical benefit per millimole of LDL-C reduced with ezetimibe in the IMPROVE-IT study was 0.80, comparable to the 0.78 observed with statins in the CTT meta-analysis.22
Efficacy Results of the IMPROVE-IT Study
| Result | Simvastatin monotherapy, % | Simvastatin + ezetimibe, % | HR (95%CI) | P |
|---|---|---|---|---|
| Primary endpoint* | 34.7 | 32.7 | 0.936 (0.89-0.99) | .016 |
| Death from any cause | 15.3 | 15.4 | 0.99 (0.91-1.07) | .78 |
| Death from cardiovascular causes | 6.8 | 6.9 | 1.00 (0.89-1.13) | 1 |
| Nonfatal MI | 14.4 | 12.8 | 0.87 (0.80-0.95) | .002 |
| Ischemic stroke | 4.1 | 3.4 | 0.79 (0.67-0.94) | .008 |
95%CI, 95% confidence interval; HR, hazard ratio; MI, myocardial infarction.
Composite of cardiovascular death, nonfatal myocardial infarction, unstable angina with rehospitalization, coronary revascularization (at least 30 days after randomization), and nonfatal stroke.
Reproduced with the permission of Cannon et al.19
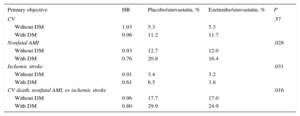
The results of the diabetes mellitus (DM) substudy of the IMPROVE-IT trial23 were presented at the 2015 European Congress of Cardiology and contrasted with the results of the overall study. The substudy compared the data of 4933 patients with DM with those of 13 202 patients without DM. Patients with DM2 in the ezetimibe/simvastatin branch had an LDL-C reduction of 43mg/dL after 1 year vs a 23mg/dL reduction in patients with DM2 in the placebo/simvastatin branch. The primary composite endpoint was reached in 40.0% of the diabetic patients taking ezetimibe/simvastatin vs 45.5% in those taking placebo/simvastatin (HR = 0.86; P = .023) (Table 3). In contrast, there was no difference in the primary endpoint in the new analysis between nondiabetics taking ezetimibe and those who received the placebo (event occurrences of 30.2% and 30.8%). There were also no differences in any of the predetermined secondary end points.
Results of the IMPROVE-IT Substudy in the Diabetic Population
| Primary objective | HR | Placebo/simvastatin, % | Ezetimibe/simvastatin, % | P |
|---|---|---|---|---|
| CV | .57 | |||
| Without DM | 1.03 | 5.3 | 5.3 | |
| With DM | 0.96 | 11.2 | 11.7 | |
| Nonfatal AMI | .028 | |||
| Without DM | 0.93 | 12.7 | 12.0 | |
| With DM | 0.76 | 20.8 | 16.4 | |
| Ischemic stroke | .031 | |||
| Without DM | 0.91 | 3.4 | 3.2 | |
| With DM | 0.61 | 6.5 | 3.8 | |
| CV death, nonfatal AMI, or ischemic stroke | .016 | |||
| Without DM | 0.96 | 17.7 | 17.0 | |
| With DM | 0.80 | 29.9 | 24.9 |
AMI, acute myocardial infarction; CV, cardiovascular; DM, diabetes mellitus; HR, hazard ratio.
Reproduced with the permission of Giugliano et al.23
The results of the study are positive for diabetic patients with ACS. The question now is whether nondiabetic patients with ACS benefit from the addition of ezetimibe. The answer probably lies in the benefit obtained in high-risk groups: patients older than 75 years, at high risk of stroke, or with advanced vascular disease.
Regarding monoclonal antibodies that inhibit the PCSK9 receptor, both alirocumab and evolocumab reduce LDL-C concentrations by between 40% and 70% in patients receiving statin therapy.
The ODYSSEY LONG TERM trial of alirocumab included 2341 patients with high CV risk (68.9% with history of coronary heart disease and 17.7% with heterozygous familial hypercholesterolemia) and LDL-C ≥ 70mg/dL on statin therapy at the maximum tolerated dose, with and without another lipid-lowering treatment (the mean baseline LDL-C was 122mg/dL). Patients were randomized 2:1 to receive alirocumab 150mg or placebo for 78 weeks. At 24 weeks, the primary efficacy endpoint showed a difference of –2% (P < .001) between alirocumab and placebo in the mean percentage change in LDL-C vs baseline; this difference remained at 78 weeks. The LDL-C target of < 70mg/dL was additionally reached by 79.3% of patients in the alirocumab group vs 8.0% in the placebo group (P < .001). Regarding safety, no significant differences were seen in the incidences of adverse events (81.0% with alirocumab and 82.5% with placebo; P = .40), adverse event-related treatment withdrawal (7.2% with alirocumab and 5.8% with placebo; P = .26), incidence of neurocognitive changes (1.2% with alirocumab vs 0.5% with placebo; P = .17), and liver enzyme elevation. However, a higher incidence of myalgia was seen in the alirocumab group (5.4% vs 2.9%; P = .006). In a post-hoc analysis, the incidence of major CV events (death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or hospitalization for unstable angina), also the prespecified primary end point of the ongoing ODYSSEY OUTCOMES (NCT01663402) trial, was lower with alirocumab than with placebo (1.7% vs 3.3%; HR = 0.52; 95% confidence interval [95%CI], 0.31-0.90; P = .02).
The OSLER study included 4465 patients who had completed 1 of the 12 phase 2 or phase 3 studies of evolocumab. Regardless of the baseline study to which they had been assigned, patients were randomized 2:1 to receive evolocumab (140mg every 2 weeks or 420mg monthly) plus standard therapy or standard therapy alone; the mean follow-up duration was 11.1 months and the study evaluated lipid concentrations, safety, and CV events (death, myocardial infarction, unstable angina, coronary revascularization, stroke, transient ischemic attack, and heart failure). At 12 weeks, evolocumab reduced the LDL-C concentration by 61% (from a mean of 120 mg/dL to 48mg/dL) vs standard therapy (P < .001). Additionally, 73.6% of patients achieved LDL-C values < 70mg/dL with evolocumab vs 3.8% with standard therapy. No significant differences were seen in adverse events (69.2% with evolocumab and 64.8% with standard therapy), although the incidence of neurocognitive events was higher with evolocumab (0.9% vs 0.3%). There were fewer CV events at 1 year with evolocumab (0.95% vs 2.18%; HR = 0.47; 95%CI, 0.28-0.78; P = .003).
DIABETES MELLITUSThis year, various relevant studies have been published in the area of antidiabetic drugs, such as the TECOS,24 ELIXA,25 and EMPA-REG OUTCOME26 trials.
The TECOS study included 14 671 patients (7332 in the sitagliptin group and 7339 in the placebo group) and compared the safety of sitagliptin with that of the “placebo”. The aim of this noninferiority study was to evaluate CV safety. It was not a typical cardiology study because the variable controlled with treatment (glycemia, glycated hemoglobin [HbA1c]) had to be equally controlled in the 2 treatment groups (sitagliptin and “placebo”, a group that was administered other lipid-lowering drugs to achieve good glycemic control). This approach allowed the study team to reject the possibility that any difference between the groups was attributable to a difference in glycemic control (which would be considered study failure) and, thus, confirm that any differences were due to the drug under investigation.
Of the previously published studies of dipeptidyl peptidase-4 inhibitors, TECOS has the longest follow-up, with lower (7.2%) baseline HbA1c levels and a DM duration > 11 years. The results have shown that, for a similar glycemic control (statistically significant but not clinically relevant differences were found), there were no differences in any of the defined CV endpoints (primary composite CV endpoint of CV death, new myocardial infarction, new stroke, and hospitalization for unstable angina) or in admissions for heart failure (defined in a similar manner to previous studies of saxagliptin27 and alogliptin28) or the incidence of pancreatitis or pancreatic neoplasms or other neoplasms, with a similarly low rate of hypoglycemia in the 2 groups. In the sitagliptin group, a reduced need for other lipid-lowering drugs was observed, as well as a longer time until the addition of new drugs and a lower rate of insulinization (and later introduction).
ELIXA is the first study of the CV safety of receptor analogues of glucagon-like peptide 1. This event-driven study included 6068 patients, with a follow-up of 2.1 years, double-blind randomized to lixisenatide or placebo, in diabetic patients in the first 180 days after ACS (unstable angina, 17%; non–ST-segment elevation acute myocardial infarction, 43%; ST-segment elevation acute myocardial infarction, 39%). The primary composite endpoint of the study was CV death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for unstable angina. Other secondary endpoints of cardiologic interest were evaluated, namely, hospitalization for heart failure, coronary revascularization, and all-cause death. The initial dose of lixisenatide of 10μg/day could be increased up to 20μg/day according to glycemic control (1 injection at breakfast). The initial statistical analysis was of noninferiority and, if satisfied, of superiority. The baseline HbA1c was 7.6%, with a DM duration of 9.4 years (with a notable percentage of patients recently diagnosed with DM during the index hospitalization for ACS). The baseline treatment and the control of CV risk factors were appropriate. A 0.27% reduction in HbA1c was seen in the lixisenatide group (statistically significant but not clinically relevant). Weight loss was 0.7kg (in 2 years; statistically significant but not clinically relevant), as well as a blood pressure reduction (fall of 0.8mmHg in systolic pressure). Heart rate increased by 0.4 bpm. The rate of hypoglycemia was similar in the 2 groups, without significant differences in the primary composite CV endpoint, secondary endpoints, number of hospitalizations for heart failure, or pancreatitis or pancreatic neoplasms or other neoplasms. More adverse gastrointestinal episodes were seen in the lixisenatide group (mainly nausea and vomiting). Patients with heart failure at baseline had a much worse prognosis in both treatment groups (4 times worse).
The EMPA-REG OUTCOME study marked a milestone in studies of oral antidiabetic agents: empagliflozin became the first oral antidiabetic to reduce CV risk in patients with DM2 and at high risk of CV events. This double-blind and multicenter study randomized 7034 patients to receive empagliflozin (at a dosage of 10 mg or 25mg once a day) or placebo. The primary endpoint was the composite outcome of CV death, nonfatal myocardial infarction, and nonfatal stroke. The secondary endpoint included hospitalization for unstable angina. The median treatment duration was 2.6 years. The primary endpoint occurred in 490 (10.5%) of the 4687 patients in the empagliflozin group and in 282 (12.1%) of the 2333 patients in the placebo group (HR = 0.86; 95%CI, 0.74-0.99; P = .04 for superiority). There were no significant differences in the incidence of acute myocardial infarction or stroke. There was a significant reduction in CV mortality in the empagliflozin group (relative risk reduction, 38%; HR = 0.62; 95%CI, 0.49-0.77). There were no differences in hospitalizations for unstable angina (HR = 0.99; 95%CI, 0.74-1.34). In the empagliflozin group, there were relative risk reductions of 35% in hospitalization for heart failure (2.7% vs 4.1%) and of 32% in all-cause death (5.7% vs 8.3%). Both 10mg and 25mg empagliflozin doses similarly reduced CV death, all-cause death, and hospitalizations for heart failure.
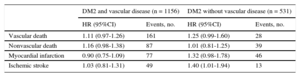
Due to the publication of the SMART study,29 the relationship between the HbA1c level and CV events is being questioned in patients with DM2, with and without established vascular disease. In this study, the relationship between HbA1c control and the incidence of new CV events and mortality were examined in 1687 patients with DM2 with and without CV disease, followed up for a mean of 6.1 years. The results showed a HR of 1.06 (95%CI, 0.97-1.17) in all patients for the relation between HbA1c and CV events. Thus, in patients with DM2, there was a small but statistically significant relation between HbA1c levels and CV events. This relationship was independent of the presence of clinical symptoms of the disease (Table 4).
Relationship Between Cardiovascular Events and Glycated Hemoglobin in Patients with Type 2 Diabetes Mellitus With and Without Established Cardiovascular Disease
| DM2 and vascular disease (n = 1156) | DM2 without vascular disease (n = 531) | |||
|---|---|---|---|---|
| HR (95%CI) | Events, no. | HR (95%CI) | Events, no. | |
| Vascular death | 1.11 (0.97-1.26) | 161 | 1.25 (0.99-1.60) | 28 |
| Nonvascular death | 1.16 (0.98-1.38) | 87 | 1.01 (0.81-1.25) | 39 |
| Myocardial infarction | 0.90 (0.75-1.09) | 77 | 1.32 (0.98-1.78) | 46 |
| Ischemic stroke | 1.03 (0.81-1.31) | 49 | 1.40 (1.01-1.94) | 13 |
95%CI, 95% confidence interval; DM2, type 2 diabetes mellitus; HR, hazard ratio. Modified with the permission of Kranenburg et al.29
In recent years, cardiac rehabilitation has evolved from a purely hospital-based approach to a mixed hospital-home monitoring strategy and even a purely home-based approach involving telephone support. This adaptation to the changing times takes advantage of effective combinations of new technologies. Thus, a recent Cochrane analysis30 comparing in-hospital rehabilitation with home-based rehabilitation in low-risk patients concluded that both approaches are equally effective. This finding supports the development of home programs for patients requiring them, instead of the classic hospital program, which could improve the degree of adherence. The development of this type of program is not only applicable to the field of ischemic heart disease, but increasingly applies to patients with heart failure and even peripheral vascular disease. In the latter field, another study also compared supervised exercise with unmonitored home-based exercise,31 concluding that supervised exercise more effectively improved the distance covered.
Another therapeutic option is the addition of monitored phase III home exercise after a phase II program. In a study32 of patients with New York Heart Association class III heart failure treated with resynchronization, the improvement was temporary in the first months and was not sustained after 12 months compared with classical phase II treatment. Telerehabilitation is promising but the evidence is still scarce.33 Nonetheless, promising results have been found with remote monitoring shirts equipped with monitoring and support software34 and even smart phone-based home monitoring.35
With these new technologies, a link could be created between preventive health consultations and cardiac rehabilitation programs. The Cardiac Rehabilitation section of the European Society of Cardiology is encouraging the creation of a comprehensive model that integrates preventive cardiology and cardiac rehabilitation to optimize long-term outcomes in patients.36
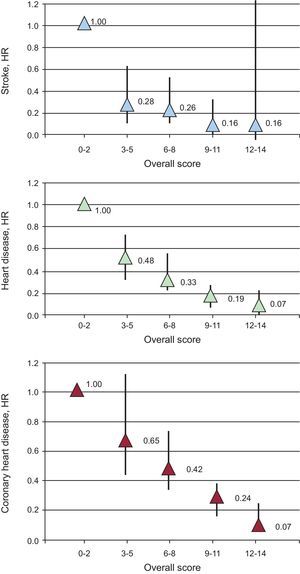
CONCLUSIONSMore and more evidence indicates that prevention should not focus solely on any single risk factor and that a pharmacological approach is insufficient for the optimal control of CV risk factors. Lifestyle changes can considerably reduce CV morbidity and mortality. The EPIC-Norfolk study37 showed that minor changes in only 7 health indicators (body mass index, diet, physical activity, smoking status, blood pressure, HbA1c, and overall cholesterol) could induce dramatic reductions in vascular risk of up to 93% (Figure 2). Thus, health care practitioners must continue to insist on educating their patients through secondary prevention programs that help them to acquire heart-healthy lifestyle habits and, consequently, achieve optimal control of CV risk factors.
Cardiovascular health score. Global score according to the EPIC-Norfolk study. The global cardiovascular health score was calculated using these 7 health indicators: body mass index; healthy diet (> 4 healthy foods/day, ideal; 3-2, intermediate; < 2, poor); physical activity (active, ideal; somewhat active, intermediate; inactive, poor); smoking status (never smoked, ideal; exsmoker, intermediate; current smoker, poor); blood pressure (< 120/80mmHg, ideal; 120-129/80-89, intermediate; > 140/90, poor); glycated hemoglobin (< 5.7%, ideal; 5.8%-6.4%, intermediate; > 6.5%, poor), and cholesterol (< 200mg/dL, ideal; 200-240, intermediate; > 240, poor). Two points were assigned to the ideal value, 1 point to the intermediate value, and 0 points to the poor value. The global cardiovascular health score was between 0 and 14 and was divided into 5 categories: 0-2 (unhealthy), 3-5, 6-8, 9-11, and 12-14 (healthy behavior). HR: hazard ratio. Modified with permission from Lachman et al.37
X. Moll Marimón: compensation from MSD for presentations and consultancy.