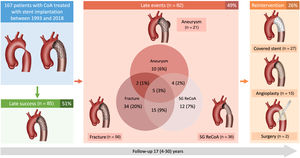
Stent implantation is the preferred treatment in older children and adults with aortic coarctation (CoA). We aimed to determine the incidence of very late events after CoA stenting.
MethodsWe analyzed a cohort of CoA patients who underwent stent implantation at our center between 1993 and 2018. Patients were periodically followed up in outpatient clinics, including computed tomography (CT) and fluoroscopy assessment.
ResultsA total of 167 patients with CT and fluoroscopy data were included: 83 (49.7%) were aged ≤ 12 years and 46 (28%) were female. The mean clinical follow-up time was 17±8 (range 4-30) years and the mean time to CT/fluoroscopy was 11±7 years. Aortic aneurysm was present in 13% and was associated with the PALMAZ stent (OR, 3.09; 95%CI, 1.11-9.49; P=.036) and the stented length (OR, 0.94; 95%CI, 0.89-0.99; P=.039). Stent fracture was frequent (34%), but was not related to the presence of aneurysm. Stent fracture was associated with young age (OR, 3.57; 95%CI, 1.54-8.33; P=.003), male sex (OR, 4.00; 95%CI, 1.51-12.5, P=.008) and inversely with the PALMAZ stent (OR, 0.29; 95%CI, 0.12-0.67, P=.005). Reintervention was lower in adults (10%), mainly related to aneurysms. Those treated when aged ≤ 12 years had higher reintervention rates (43%) due to recoarctation somatic growth.
ConclusionsThis long-term follow-up study of CoA patients treated with stenting revealed a significant incidence of late events. Reintervention rates were higher in patients treated at younger ages. Periodic imaging surveillance appears to be advisable.
Keywords
According to the recommendations of the European guidelines,1–3 stent implantation is the treatment of choice in older children and adults with significant aortic coarctation. However, since late complications after a successful procedure have been reported,4 close follow-up of these patients seems to be advisable.5 Despite current evidence documenting the occurrence of aortic aneurysm years after coarctation stenting, its true incidence and clinical impact are difficult to determine due to the lack of a universally accepted definition6 and the scarcity of long-term follow-up studies with serial computed tomography (CT) assessment.
The aim of the present study was to determine the incidence of late events after aortic stenting and their management, as well as to identify the factors associated with these complications.
METHODSStudy design and populationWe analyzed consecutive children and adults with aortic coarctation who underwent stent implantation at a single center between 1993 and 2018. In all, 177 patients were eligible. Patients with postsurgery (before stenting) aneurysm, acute aortic wall damage during the procedure, and those with no available follow-up CT or fluoroscopy studies were excluded from the study. Thus, the final cohort consisted of 167 patients (figure 1). The study was conducted according to the Declaration of Helsinki and was approved by our clinical research ethics committee. Written informed consent was obtained from all participants/parents/legal guardians.
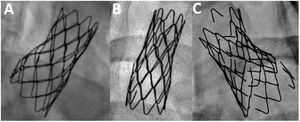
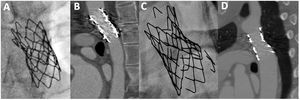
Endpoints and definitionsThe main endpoint of the study was the incidence of aneurysm and stent fracture at long-term follow-up. Secondary endpoints were the need for reintervention and the occurrence of other major events (stent migration, all-cause mortality). Additionally, we aimed to identify clinical and procedural factors associated with these complications. Aneurysms were classified according to Pedra et al.7 as small (> 3mm and ≤ 50% the diameter of the descending aorta at the level of the diaphragm), moderate (≥ 50% of the diameter of the aorta) and large (≥ 50mm). Stent fractures were categorized according to a modified McElhinney evaluation.8 Thus, we considered that a stent fracture was mild when 1 or 2 struts were detached with a separation ≤ 2mm (figure 2A), moderate if multiple struts were detached with a separation ≤ 2mm (figure 2B), or severe when multiple struts were detached with a separation >2mm or there was a significant displacement of stent fragments (figure 2C). Fractures were evaluated after aortic stent implantation before any type of reintervention. Thus, stent fractures caused by balloon redilation during the follow-up were not included. Primary data were reviewed by 2 independent expert operators.
Stent implantation procedureThe procedure of stent implantation has been previously described in detail.9 In brief, 2 femoral or radial/femoral access were obtained. Simultaneous peak-to-peak pressure gradient across the coarctation segment was measured before and after treatment. Angiography was performed to assess the anatomy of the aorta and the stented segment. The minimal lumen diameter at the coarctation level, the isthmus diameter at the level of the left subclavian artery, and the aortic diameter at the level of the diaphragm were measured. After diagnostic catheterization, the stent size was selected according to the diameter of the aorta at the level of the diaphragm. The type of stent varied over the years according to the availability of the different models at the time of the treatment: PALMAZ (Cordis Endovascular, United States), Cheatham-Platinum (CP) (NuMED, United States), Valeo (Bard Peripheral Vascular, United States), BeGraft (Bentley InnoMed, Germany). Since 2011, the femoral puncture was closed by a Prostar or a Proglide devices (Abbott Vascular Inc., United States) (previously implanted before the cannula insertion).
Follow-upFollow-up visits included telephone calls and scheduled clinical and echocardiographic evaluations at 6 months, 1 year, and each subsequent year. CT scan surveillance was performed from 2008 onwards (at least 1 study was available from each of the included patients). Additionally, a fluoroscopy study with several projections was performed to identify stent fractures. Major events, the presence of an aortic aneurysm, stent fracture, stent migration, and the need for reintervention were ascertained.
Statistical analysisCategorical data are presented as counts (percentages) and continuous data as mean±standard deviation. Comparisons between groups were made using the chi-square test or the Fisher exact test for categorical variables and the Student t-test or the Mann-Whitney U test for continuous variables. Univariable and multivariable logistic regression models were used to study the factors associated with aneurysm and stent fracture. To evaluate the risk of reintervention and all-cause death, time-to-event analyses were conducted using Kaplan-Meier curves and univariable and multivariable Cox proportional-hazards models. Logistic and Cox multivariable models were tested for collinearity and were built using backward stepwise elimination, initially including clinically relevant variables and those with a P<.100 in the univariable models. All tests were 2-tailed and were considered significant when P<.05. Statistical analyses were performed using SPSS software (version 24; IBM Corp, Armonk, NY, USA) and R software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria).
RESULTSBaseline, angiographic, and procedural dataThe baseline clinical, angiographic, and procedural data of the overall cohort categorized according to age less or greater than 12 years old are shown in table 1. Obviously, there were significant differences in terms of age and aortic sizes between groups. With respect to the procedural data, a high proportion of patients in both groups were treated with a PALMAZ stent at the first procedure [76 (45.5%)] and the use of covered stents was rare (11%) according to the late availability of this dedicated stent during the study period.
Baseline data
| Characteristic | OverallN=167 | ≤ 12 yn=83 | >12 yn=84 | P |
|---|---|---|---|---|
| Clinical | ||||
| Age, y | 19±15 | 7±3 | 31±14 | <.001 |
| Female sex, % | 46 (27.5) | 18 (21.7) | 28 (33.3) | .092 |
| Body surface area, m2 | 1.4±0.5 | 1.0±0.4 | 1.7±0.3 | <.001 |
| Previous coarctation procedure | ||||
| Balloon | 25.0 (15.0) | 18.0 (21.7) | 7.0 (8.3) | .016 |
| Surgery | 25.0 (15.0) | 13.0 (15.7) | 12.0 (14.3) | .803 |
| Associated malformations | 54.0 (32.3) | 30.0 (36.1) | 24.0 (28.6) | .296 |
| Angiographic | ||||
| Ascending aortic size, mm | 23.9±12.7 | 16.2±5.1 | 31.1±13.5 | <.001 |
| Descending aortic size, mm | 16.8±6.5 | 12.8±3.5 | 20.9±6.4 | <.001 |
| Aortic minimal lumen diameter, mm | 5.7±3.9 | 4.7±3.0 | 6.6±4.4 | .007 |
| Aortic arch, mm | 15.2±7.6 | 11.3±4.2 | 18.8±8.3 | <.001 |
| Aortic size (post subclavian), mm | 13.4±6.1 | 10.0±4.6 | 16.6±5.6 | <.001 |
| Coarctation stenosis, % | 66.7±20.5 | 65.6±19.5 | 67.7±21.5 | .573 |
| Peak gradient, mmHg | 38.03±14.08 | 39.34±15.10 | 36.79±13.02 | .356 |
| Procedural | ||||
| Noncovered stent | 145 (86.8) | 77 (92.8) | 68 (81.0) | .024 |
| PALMAZ stent | 76 (45.5) | 37 (44.6) | 39 (46.4) | .810 |
| Cheatham-Platinum stent | 77 (46.1) | 42 (50.6) | 35 (41.7) | .247 |
| Other stents | 14 (8.4) | 4 (4.8) | 10 (11.9) | .099 |
| Stent diameter | 16.9±5.6 | 14.2±3.5 | 20.2±6.0 | <.001 |
| Stent length | 39.9±13.5 | 35.2±8.1 | 44.6±15.9 | <.001 |
The data are expressed as No. (%) or mean±standard deviation.
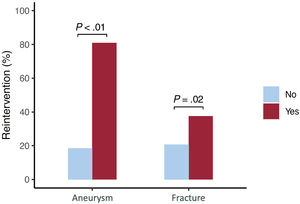
The prevalence of aortic aneurysms after coarctation stenting was 13% (figure 1). The mean time between stent implantation and the last CT was 11±8 years. Twelve aneurysms were observed in the group of younger patients (14%), while 9 were detected in the group of patients older than 12 years (11%), with no significant differences between the groups (table 2). Aneurysm location was at the proximal stent border in 8 (38%), at the stent body in 9 (43%), and at the distal stent border in 4 (19%) patients. The only factors independently associated with this late complication were the stented length and the use of a PALMAZ stent (table 3). When patients treated with covered stent were excluded, the PALMAZ stent showed a tendency toward a higher likelihood of aneurysm formation (19% vs 8.7%; P=.06). The severity of the aortic coarctation in terms of baseline gradient showed a trend toward significance in the univariable study. Most of the aneurysms were classified as small or moderate (table 2), and only 3 (14%) were large (figure 3). Once an aneurysm was detected, it was treated with a new covered stent in most patients [16 (76%)] (figure 4). Accordingly, there was a close relationship between aneurysm and reintervention (figure 5). One patient with a large aneurysm and stent migration was surgically treated (figure 3). The remaining 4 patients (3 young and 1 adult patient) with small aneurysms are being closely followed in outpatient clinics (Figure 1 of the supplementary data).
Late follow-up events
| TotalN=167 | ≤12 yn=83 | >12 yn=84 | P | |
|---|---|---|---|---|
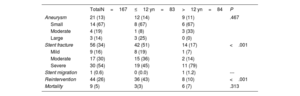
| Aneurysm | 21 (13) | 12 (14) | 9 (11) | .467 |
| Small | 14 (67) | 8 (67) | 6 (67) | |
| Moderate | 4 (19) | 1 (8) | 3 (33) | |
| Large | 3 (14) | 3 (25) | 0 (0) | |
| Stent fracture | 56 (34) | 42 (51) | 14 (17) | <.001 |
| Mild | 9 (16) | 8 (19) | 1 (7) | |
| Moderate | 17 (30) | 15 (36) | 2 (14) | |
| Severe | 30 (54) | 19 (45) | 11 (79) | |
| Stent migration | 1 (0.6) | 0 (0.0) | 1 (1.2) | --- |
| Reintervention | 44 (26) | 36 (43) | 8 (10) | <.001 |
| Mortality | 9 (5) | 3(3) | 6 (7) | .313 |
The data are presented as No. (%).
Predictors of aortic aneurysm. Univariable and multivariable logistic regression models
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
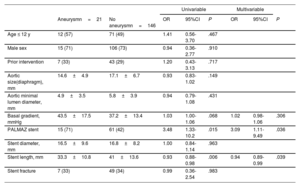
| Aneurysmn=21 | No aneurysmn=146 | OR | 95%CI | P | OR | 95%CI | P | |
| Age ≤ 12 y | 12 (57) | 71 (49) | 1.41 | 0.56-3.70 | .467 | |||
| Male sex | 15 (71) | 106 (73) | 0.94 | 0.36-2.77 | .910 | |||
| Prior intervention | 7 (33) | 43 (29) | 1.20 | 0.43-3.13 | .717 | |||
| Aortic size(diaphragm), mm | 14.6±4.9 | 17.1±6.7 | 0.93 | 0.83-1.02 | .149 | |||
| Aortic minimal lumen diameter, mm | 4.9±3.5 | 5.8±3.9 | 0.94 | 0.79-1.08 | .431 | |||
| Basal gradient, mmHg | 43.5±17.5 | 37.2±13.4 | 1.03 | 1.00-1.06 | .068 | 1.02 | 0.98-1.06 | .306 |
| PALMAZ stent | 15 (71) | 61 (42) | 3.48 | 1.33-10.2 | .015 | 3.09 | 1.11-9.49 | .036 |
| Stent diameter, mm | 16.5±9.6 | 16.8±8.2 | 1.00 | 0.84-1.14 | .963 | |||
| Stent length, mm | 33.3±10.8 | 41±13.6 | 0.93 | 0.88-0.98 | .006 | 0.94 | 0.89-0.99 | .039 |
| Stent fracture | 7 (33) | 49 (34) | 0.99 | 0.36-2.54 | .983 | |||
95%CI, 95% confidence interval; OR, odds ratio.
The data are presented as No. (%) or mean±standard deviation.
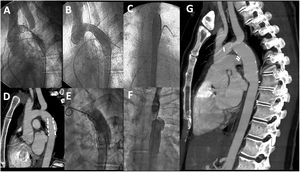
Example of late aneurysm formation and stent migration evidenced 18 years after successful stent implantation. A, baseline angiography; B and C, immediate result: LAO aortography and PA aortography, respectively; D, CT 18 years after stent implantation; E and F, LAO and PA angiography at the time of the CT; G, CT after surgical repair. CT, computed tomography; LAO, left anterior oblique view; PA, posteroanterior view.
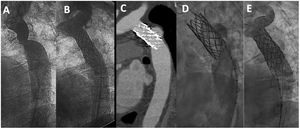
Example of late aneurysm formation evidenced 15 years after successful stent implantation. A, baseline angiography; B, immediate result; C, CT 15 years after stent implantation; D, angiography at the time of the CT; E, exclusion of the aneurysm after covered stents implantation. CT, computed tomography.
Stent fracture was a frequent event that occurred in 56 patients (34%) after a mean time since the index procedure of 11±7.4 years (figure 1). Most fractures were moderate or severe (table 2). Stent fractures occurred more frequently in younger patients, males and patients not receiving a PALMAZ stent (table 4). Patients with smaller aortic sizes and shorter stented lengths showed a higher incidence of stent fractures in the univariable study that lost significance in the multivariable analysis. The presence of stent fractures was not related to the development of aneurysms (table 3). Figure 6 shows 2 examples of patients with aortic wall integrity despite severe stent fractures. We observed an association between stent fracture and the need for reintervention (figure 5). This association seemed to be related to the high incidence of stent fractures in younger patients (table 4), most of whom needed a second intervention after completion of somatic growth.
Predictors of stent fracture. Univariable and multivariable logistic regression models
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
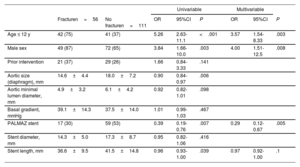
| Fracturen=56 | No fracturen=111 | OR | 95%CI | P | OR | 95%CI | P | |
| Age ≤ 12 y | 42 (75) | 41 (37) | 5.26 | 2.63-11.1 | <.001 | 3.57 | 1.54-8.33 | .003 |
| Male sex | 49 (87) | 72 (65) | 3.84 | 1.66-10.0 | .003 | 4.00 | 1.51-12.5 | .008 |
| Prior intervention | 21 (37) | 29 (26) | 1.66 | 0.84-3.33 | .141 | |||
| Aortic size (diaphragm), mm | 14.6±4.4 | 18.0±7.2 | 0.90 | 0.84-0.97 | .006 | |||
| Aortic minimal lumen diameter, mm | 4.9±3.2 | 6.1±4.2 | 0.92 | 0.82-1.01 | .098 | |||
| Basal gradient, mmHg | 39.1±14.3 | 37.5±14.0 | 1.01 | 0.99-1.03 | .467 | |||
| PALMAZ stent | 17 (30) | 59 (53) | 0.39 | 0.19-0.76 | .007 | 0.29 | 0.12-0.67 | .005 |
| Stent diameter, mm | 14.3±5.0 | 17.3±8.7 | 0.95 | 0.82-1.06 | .416 | |||
| Stent length, mm | 36.6±9.5 | 41.5±14.8 | 0.96 | 0.93-1.00 | .039 | 0.97 | 0.92-1.00 | .1 |
95%CI, 95% confidence interval; OR, odds ratio.
The data are expressed as No. (%) or mean±standard deviation.
Although this complication may occur during the stent implantation procedure, it is much less frequent during follow-up. In our series, only 1 adult patient (0.6%) who developed a large aneurysm experienced this complication (figure 3). The mechanism was probably related to a lack of aortic support due to wall dilation. The patient underwent surgery, the stent was removed, and the aneurysm was resected (figure 3).
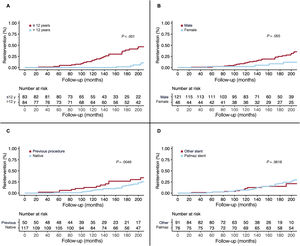
Need for reinterventionReintervention during the follow-up period (17±8 years) occurred in 44 (26%) patients. Children younger than 12 years were treated with a first stenting procedure while accepting the need for a second procedure after the completion of their somatic growth.9 Therefore, in this group of patients, the reintervention rate was high (43%). In contrast, in the adult group of patients, the reintervention rate was much lower (10%) and was only due to the presence of aortic aneurysms. Since the latter situation was rare, there were significant differences in terms of reinterventions between the group of children younger than 12 years and patients older than 12 years (table 5 and figure 7). In addition to age at first stent implantation, sex, and baseline gradient across the coarctation, other factors were associated with the need for reintervention (table 5 and figure 7). The need for reintervention was not influenced by technical aspects, such as the type of implanted stent (figure 7). The type of reintervention is described in figure 1: 15 (34%) patients were percutaneously treated with balloon angioplasty, 27 (61%) with covered stents, and 2 (5%) patients with surgery.
Predictors of reintervention. Univariable and multivariable Cox regression models
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
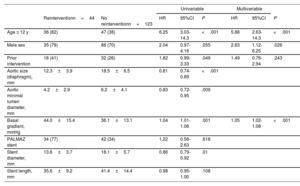
| Reinterventionn=44 | No reinterventionn=123 | HR | 95%CI | P | HR | 95%CI | P | |
| Age ≤ 12 y | 36 (82) | 47 (38) | 6.25 | 3.03-14.3 | <.001 | 5.88 | 2.63-14.3 | <.001 |
| Male sex | 35 (79) | 86 (70) | 2.04 | 0.97-4.16 | .055 | 2.63 | 1.12-6.25 | .026 |
| Prior intervention | 18 (41) | 32 (26) | 1.82 | 0.99-3.33 | .049 | 1.49 | 0.76-2.94 | .243 |
| Aortic size (diaphragm), mm | 12.3±3.9 | 18.5±6.5 | 0.81 | 0.74-0.89 | <.001 | |||
| Aortic minimal lumen diameter, mm | 4.2±2.9 | 6.2±4.1 | 0.83 | 0.72-0.95 | .009 | |||
| Basal gradient, mmHg | 44.0±15.4 | 36.1±13.1 | 1.04 | 1.01-1.06 | .001 | 1.05 | 1.02-1.08 | <.001 |
| PALMAZ stent | 34 (77) | 42 (34) | 1.22 | 0.56-2.63 | .618 | |||
| Stent diameter, mm | 13.6±3.7 | 18.1±5.7 | 0.86 | 0.79-0.92 | .01 | |||
| Stent length, mm | 35.6±9.2 | 41.4±14.4 | 0.98 | 0.95-1.00 | .108 | |||
95%CI, 95% confidence interval; OR, odds ratio.
The data are expressed as No. (%) or mean±standard deviation.
Nine patients (5%) died during the follow-up period (17±8 years). In this study, we could not identify predictors of mortality. Sex, group of age, type of stent or a previous intervention on the aortic coarctation were not related to all-cause of death (figure 8). Causes of death were: 1 cancer, 1 brain abscess, 1 viral encephalitis, 2 sudden deaths, 1 heart failure while waiting for a heart transplant, 1 aneurysm of the ascending aorta, 1 abdominal bleeding, and 1 massive hemoptysis after a traffic accident.
DISCUSSIONThe main findings of our study are as follows: a) aortic aneurysm after successful stent implantation in coarctation of the aorta occurred in 13% of our patients at 11±8 years follow-up. The only factors associated with this late complication were the use of the PALMAZ stent and the stented length; b) stent fracture was a frequent event (34%), but was not related to the presence of aneurysms. Stent fractures occurred more frequently in younger patients, male patients, and in those who did not receive a PALMAZ stent; c) the reintervention rate was low (10%) and was mainly related to the presence of aneurysms. Younger patients receiving a stent at age less than 12 years had a high reintervention rate (43%) due to the need to adapt the stent lumen to the final size of the aorta; and d) all-cause mortality was low (5%), despite the long-term follow-up.
Aortic aneurysm after stenting for coarctation of the aortaSince the first descriptions,10,11 late aortic aneurysm has been a recognized complication of stent placement in aortic coarctation. However, its true incidence is difficult to establish due to the scarcity of series with serial imaging examinations during a long-term follow-up. Thus, wide variability in the incidence of aneurysm has been reported: some series with short-term follow-up (2-3 years) have reported no cases of aneurysm,12,13 while series with longer follow-up (2-6 years) have described an incidence of aortic aneurysm that ranges between 1.3 and 9%.14–22 In our study, the incidence of aortic aneurysm was higher than previously reported (13%). The reasons for this finding seem to be a longer follow-up, the routine use of imaging techniques following stent implantation and the frequent use of the stainless steel PALMAZ stent. We do not have a definitive explanation for this finding. However, it may be hypothesized that the lesser flexibility of this first-generation stent as well as the occurrence of “dog-boning” or asymmetric shortening during expansion, may favor wall damage. From a theoretical point of view, covered stents could reduce the incidence of this complication.23–29 Thus, several series25–29 have reported the absence of aneurysms at short-term follow-up (1-3 years). However, in a randomized study30 comparing covered vs bare metal stents, the incidence of aneurysm was higher in the group of patients treated with covered stents (3.3% vs 0%). Despite this disappointing finding, covered stents seem to be the current logical approach for many adult patients with coarctation of the aorta to prevent aneurysm formation. The disadvantages of covered stents include the need for a larger femoral sheath leading to a higher rate of complications at the access site31 and the possibility of occlusion of an important side branch proximal to the coarctation segment. Most of our observed aneurysms were small; although the significance of these small aortic aneurysms is not well-known, until proven otherwise, it seems prudent to approach them as clinically important.20 Following this philosophy, we treated most of them by implanting a covered stent.32 According to our findings, we propose an image technique based on periodic follow-up, adapted from the 2018 ACC/AHA guidelines5: starting 2-3 years after stent implantation, and then every 3 years in the absence of aneurysm. If moderate-severe aneurysms appear, a reintervention is recommended. For small aneurysms, a decision between reintervention or a close follow-up (1-2 years) (Figure 1 of the supplementary data) should be evaluated on an individual basis.
Stent fracturesStents implanted at the aorta are subjected to mechanical fatigue throughout the years, which gradually alters the ability of the material to resist the external load.8 Factors that can affect fatigue-life include the material (eg, stainless steel, platinum-iridium, cobalt chromium), the geometry and design (eg, cut tube, welded wire, open vs closed cell, and thickness of struts), and the manufacturing process (eg, laser cut tube, welded wire, or brazing of welds).8 In our study, the CP stent showed the highest rate of fracture, but fracture was also frequently observed in the PALMAZ stent (figure 2). Some authors state that stent fractures can be associated with aortic wall damage at the level of these fractures and recommend the implantation of another stent.19 This association could not be demonstrated in our study (table 3) and wall integrity can be observed despite the presence of severe fractures (figure 6). Another important aspect of our analysis is the observed high incidence of stent fractures (34%). In a long-term analysis of the COAST I and II studies,22 the authors found a stent fracture rate of 24% at 5 years follow-up, while Boe et al.33 described a rate of 21% at 75 months of follow-up in children. These differences could be explained by the high frequency of fluoroscopic studies that were performed during a long-term follow-up.
ReinterventionThe reasons for reintervention after successful previous stent implantation in aortic coarctation may include restenosis, undersized stent because of somatic growth, stent underexpansion, aneurysm formation, and stent migration. In our study, most reinterventions in adult patients were due to the presence of aortic aneurysms, while in children aged ≤ 12 years a reintervention was required to adapt the stented lumen to the aortic size after completion of somatic growth. Stent fractures per se should not be a reason for reintervention unless there is recoarctation or aneurysm. However, we found an association between stent fractures and reintervention (figure 6). The explanation for this finding seems to be the high incidence of fractures (51%) occurring in children who required a second intervention due to growth-related recoarctation (size mismatch between the stented segment and the proximal and distal aortic segments). In previous recent series, as in our study, early age at the first procedure was the strongest predictor of reintervention, since most of these children had a planned staged procedure.9,31,33
LimitationsFirst, frequent serial CT scans would have been required to determine the exact timing and significance of aortic aneurysms. Although all the included patients had, at least, a long-term CT study, serial systematic studies were not available in all patients. Second, since our experience started at 1995, many of the stents used in our analysis belonged to the first generation. Many improvements in stent design have taken place over these years and were incorporated into our clinical practice once the devices were available. The analysis of the results including both the old and new designs constitutes a limitation, but, at the same time, permits long-term follow-up, which is required to achieve solid conclusions about the percutaneous treatment of coarctation of the aorta.
CONCLUSIONSAortic aneurysm and stent fracture after successful stent implantation in coarctation of the aorta were frequent events at long-term follow-up. Reintervention was infrequent in adults (10%) and was mainly related to the presence of aneurysms. Younger people receiving a stent aged less than 12 years had a higher reintervention rate (43%) due to recoarctation somatic growth. All-cause death was low (5%) despite the long-term follow-up.
Some patients with aortic coarctation successfully repaired with stent implantation may develop late complications that can be percutaneously treated.
WHAT DOES THIS STUDY ADD?Late complications are frequent in the long-term follow-up of patients with successful percutaneous repair of coarctation of the aorta. Stent fracture and aneurysm are not associated. Thus, lifetime periodic multimodality imaging surveillance (fluoroscopy, CT) is advisable to improve the clinical care of these patients.
No funding.
ETHICAL CONSIDERATIONSThe study was conducted according to the Declaration of Helsinki and was approved by our clinical research ethics committee. Written informed consent was obtained from all participants/parents/legal guardians. Sex/gender biases have been taken into account in the preparation of this article.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence has not been used.
AUTHORS’ CONTRIBUTIONSM. Pan and C. Pericet contributed equally to the present work as first authors. Conceptualization: M. Pan, S. Ojeda, M. Romero. Methodology: M. Pan, C. Pericet, R. González-Manzanares, S. Ojeda. Formal analysis: C. Pericet, R. González-Manzanares. Investigation: C. Pericet, R. González-Manzanares, M.A Díaz, J. Suárez de Lezo, F. Hidalgo, M. Alvarado, G. Dueñas, E. Gómez, S. Espejo, J. Perea. Resources: M. Pan, S. Espejo, M. Romero, S. Ojeda. Data curation: C. Pericet, R. González-Manzanares. Writing-original draft: M. Pan, C. Pericet. Writing-review and editing: R. González-Manzanares, S. Ojeda. Supervision: S. Ojeda.
CONFLICTS OF INTERESTThe authors have no conflicts of interest to declare.