Bayés syndrome is a new clinical entity, characterized by the association of advanced interatrial block (IAB) on surface electrocardiogram with atrial fibrillation (AF) and other atrial arrhythmias. This syndrome is associated with an increased risk of stroke, dementia, and mortality. Advanced IAB is diagnosed by the presence of a P-wave ≥ 120ms with biphasic morphology (±) in inferior leads. The cause of IAB is complete Bachmann bundle blockade, leading to retrograde depolarization of the left atrium from areas near the atrioventricular junction. The anatomic substrate of advanced IAB is fibrotic atrial cardiomyopathy. Dyssynchrony induced by advanced IAB is a trigger and maintenance mechanism of AF. This alteration of the atrial architecture produces atrial remodeling, blood stasis and hypercoagulability, triggering the thrombogenic cascade. The presence of advanced IAB, even in patients without documented atrial arrhythmias, has also been associated with AF, stroke, dementia, and mortality. However, in these patients, there is no evidence to support the use of anticoagulation. Therefore, in patients with advanced IAB, a proactive search for AF is recommended.
Keywords
In 1988, Bayés de Luna et al.1 reported that patients with advanced interatrial block (IAB) presented with supraventricular arrhythmia more often than patients with partial IAB. However, until a consensus article was published on IAB in 2012,2 only a few authors had shown interest in the subject, mainly the groups led by Spodick,3 García-Cosío,4 and Platonov,5 as well as our own group.6,7 Since this consensus article was published, there has been growing interest, and Conde and Baranchuk8 named this combination “Bayés syndrome,” a term quickly accepted by the scientific community.9–11
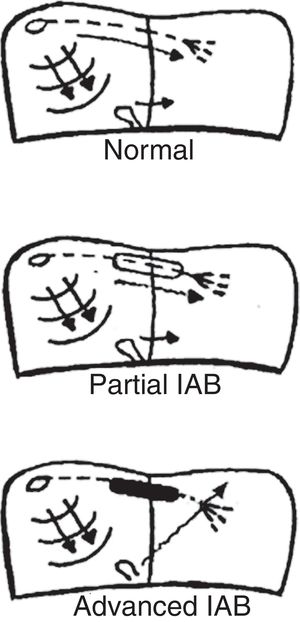
ATRIAL ACTIVATIONThe P wave originates in the sinus node, producing an atrial activation sequence that is directed downward and leftward in the frontal plane, with counterclockwise rotation, producing a P wave that is positive in leads II and aVF, variable in III and aVL, and negative in aVR. Impulses are conducted from the sinus node to the atrioventricular node without genuine bundles, whereas impulses are conducted from the right to the left atrium mainly through the upper portion of the atrium through the Bachmann bundle or region, the main conduction route between the 2 atria. The final portion of this bundle bifurcates, then encircling the neck of the atrial appendage.12,13 In the posterior and inferior aspects of the septum, almost 30% of patients also show fibers that can conduct the impulse from the right to the left atrium.5 However, the rest of the septum consists of connective tissue and does not allow impulse transmission. Consequently, if there is complete AV block of the Bachmann region, left atrial activation is retrograde from the area near the atrioventricular junction (coronary sinus and fossa ovalis), with the resulting abnormality seen on electrocardiography (P±in lower leads). As in other types of cardiac blocks, IAB may be transient, may be induced experimentally,14,15 and may be present in the absence of structural heart disease, in which case it is seen as left atrial enlargement.
TYPES OF INTERATRIAL BLOCK AND ELECTROCARDIOGRAPHY CRITERIAThe type of atrial pacing determines the type of IAB (figure 1). Therefore, we have:
- •
Partial IAB. Impulses are conducted from the right atrium to the left atrium by the Bachmann bundle, but with a delay.
- •
Advanced IAB. Impulses are not conducted by the Bachmann bundle. Left atrial activation is retrograde through the coronary sinus musculature and the fossa ovalis.
Intermittent IAB is also possible, as impulses are sometimes blocked and sometimes conducted in a single electrocardiogram tracing. Additionally, IAB may be progressive and, over the years, worsen from partial to advanced.
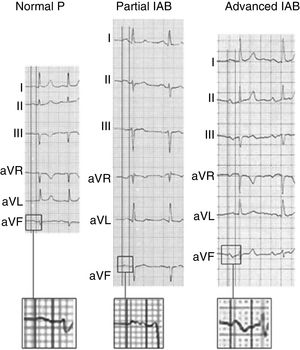
To diagnose IAB, the surface electrocardiogram should show P wave ≥ 120ms and morphology allowing the degree of blockage to be diagnosed2,16–18 (figure 2):
- •
Partial IAB: positive P wave in lower leads, often bimodal (“notched”) in some leads of the frontal and horizontal planes.
- •
Advanced IAB: biphasic P wave (±) in lower leads.
Examples of normal P wave and partial and advanced interatrial block (IAB). Adapted with permission from Martínez-Sellés et al.18
We recently described other atypical morphologies of advanced IAB19 not discussed in the present review, as they are rare and do not currently seem to be clinically relevant.
PATHOPHYSIOLOGY AND CONSEQUENCES OF ADVANCED INTERATRIAL BLOCKBoth AF and advanced IAB have fibrotic atrial cardiomyopathy as their anatomic substrate20 which, along with atrial dyssynchrony21 in patients with advanced IAB, result in left atrial hypocontractility that favors blood stasis and encourages atrial remodeling. Under these circumstances, through thrombin activation by protease-activated receptors, a hypercoagulable state follows that further increases fibrosis and atrial remodeling and triggers the clotting cascade and the appearance of systemic embolization.22
Magnetic resonance imaging is the technique of choice to detect fibrotic atrial cardiomyopathy,23 the anatomopathologic substrate for most cases of advanced IAB and AF. Patients with advanced IAB may exhibit a high degree of fibrosis, even without documented AF.24 Speckle-tracking echocardiography also can be used to assess atrial fibrosis, with prognostic implications for AF recurrence.25–27
Until recently, AF was considered the final cause leading to systemic embolization. However, several studies in patients with an implanted Holter device have not found a temporal relationship between episodes of paroxysmal atrial fibrillation and the onset of stroke.28–30 Therefore, AF is only one risk factor, just like advanced IAB and other factors such as age, hypertension, diabetes, and obesity. Hence, the importance of atrial fibrosis and its relationship with the appearance of stasis, which encourages the formation of thrombi in the left atrium and is present in both AF and advanced IAB. In fact, the novel concept of atrial failure31 includes atrial fibrosis, often in combination with atrial dilatation, interatrial conduction disorders, and thus Bayés syndrome among them.
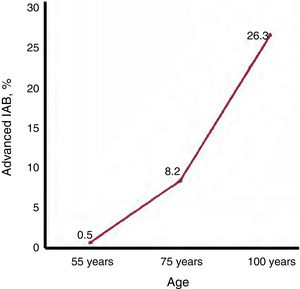
EPIDEMIOLOGY OF ADVANCED INTERATRIAL BLOCKThe prevalence of advanced IAB in the overall middle-aged population (45-64 years) is only 0.5%,32 but rises to 8.2% in septuagenarians and 26.3% in centenarians18 (figure 3). The age-related association is even more pronounced than suggested by these figures because, as AF also increases with age, the prevalence of advanced IAB in patients who remain in sinus rhythm is higher than the overall figures.
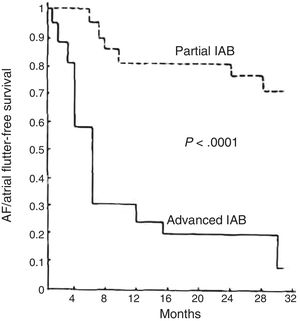
ADVANCED INTERATRIAL BLOCK AND RISK OF ATRIAL FIBRILLATION, STROKE, AND DEATHIn middle-aged patients, advanced IAB results in a 3-fold risk of AF and an almost 2-fold risk of stroke,32,33 thus providing an explanation for about half the cases of AF and a quarter of the strokes occurring in this age bracket. P-wave duration is also associated with cardiovascular mortality34 and sudden cardiac death.35 At very advanced ages, the presence of IAB is also associated with total mortality.18 Since an association between advanced IAB and supraventricular arrhythmias was first described1 (figure 4), advanced IAB has been reported as associated with prognosis in various clinical situations1,18,32,33,36–60 (table 1) and AF has been confirmed as associated with stroke in a meta-analysis.61
Supraventricular tachyarrhythmia (atrial fibrillation [AF]/atrial flutter)-free survival in patients with advanced interatrial block (IAB) compared with a similar group of patients with partial IAB in the first article to describe this association. Adapted with permission from Bayés de Luna et al.1
Studies reporting an association between advanced interatrial block and prognosis (atrial fibrillation, stroke, and mortality)
| Clinical situation | References |
|---|---|
| General population | O’Neal et al.32 (2016), O’Neal et al.33 (2016), Massó-van-Roessel et al.36 (2017) |
| Primary care | Skov et al.37 (2018) |
| Centenarians | Martínez-Sellés et al.18 (2016) |
| Pharmacologic cardioversion of atrial fibrillation | Enriquez et al.38 (2014) |
| Cavotricuspid isthmus ablation | Enriquez et al.39 (2015) |
| Atrial fibrillation ablation | Caldwell et al.40 (2014), Wu et al.41 (2016), Gul et al.42 (2017) |
| Wolff-Parkinson-White ablation | Wu et al.43 (2019) |
| Coronary and carotid disease | Alexander et al.44 (2018) |
| Acute coronary syndrome | Alexander et al.45 (2017), Bernal et al.46 (2018), Çinier et al.47 (2018), Bruna et al.48 (2019) |
| Tako-tsubo | Martín-Demiguel et al.49 (2019) |
| Heart failure | Sadiq et al.50 (2015), Escobar-Robledo et al.51 (2018) |
| Cardiac surgery | Martínez-Sellés et al.52 (2017) |
| Noncardiac surgery | Lacalzada-Almeida et al.53 (2019) |
| Chagas cardiomyopathy | Enriquez et al.54 (2014) |
| Valve disease and cardiomyopathy | Bayés de Luna et al.1 (1988) |
| Stroke | Ariyarajah et al.55 (2007), Cotter et al.56 (2013), Baturova et al.57 (2019), García-Talavera et al.58 (2019) |
| Hospitalized patients | Wu et al.59 (2016) |
| Sleep apnea | Yeung et al.60 (2018) |
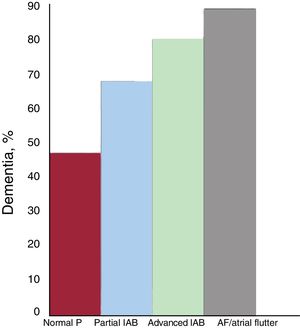
The association of AF with mild cognitive decline and dementia is no longer questioned.62 Although the pathophysiologic mechanisms explaining this association are not yet fully elucidated, they are likely to be multifactorial and to include the most obvious (eg, symptomatic ischemic stroke and silent strokes/microstrokes) as well as brain bleeds and brain hypoperfusion due to hemodynamic impairment that lowers cardiac output and reduces diastolic arterial flow to the brain. In the case of advanced IAB, the association appears to be very similar. In the Cardiac and Clinical Characterization of Centenarians (4C) study,18 the prevalence of dementia was progressively higher as normal P waves developed into partial IAB, advanced IAB, and AF (figure 5). This association is likely due to silent strokes, although other factors such as chronic cerebral hypoperfusion may play a role. This relationship between advanced IAB and dementia indicates that routine cognitive screening is needed among patients with advanced IAB. Advanced IAB should also be ruled out in patients with cognitive impairment.
Prevalence of dementia in the 4C registry,18 according to the presence and type of interatrial block (IAB) and atrial fibrillation (AF)/atrial flutter.
Patients with advanced IAB and prior episodes of documented AF (Bayés syndrome) should be treated like other patients with a history of AF. In terms of strategy, the presence of advanced IAB is an independent predictor for AF recurrence and, therefore, heart rate control could be considered in some patients.
Patients with advanced IAB and no prior episodes of documented AF also have a higher risk of stroke, particularly if there are additional risk factors, such as advanced age, diabetes, hypertension, structural heart disease, and frequent supraventricular extrasystole. However, no ongoing clinical trials support the use of anticoagulants in the absence of documented AF. Therefore, patients should be monitored for AF episodes which could require anticoagulant therapy.63 We believe it would be useful to conduct a placebo-controlled, randomized study on direct-action oral anticoagulants with patients who have advanced IAB as well as some of the other risk factors reported.64–66 It may also be useful to investigate how atrial fibrosis may be reduced with antifibrotic drugs.
CONCLUSIONSP waves do not usually draw the attention of clinicians during electrocardiogram evaluation. However, the diagnosis of advanced IAB is not merely of academic interest, as it is associated with supraventricular arrhythmia (Bayés syndrome), stroke, mortality, and dementia.
CONFLICTS OF INTERESTNone declared.




![Supraventricular tachyarrhythmia (atrial fibrillation [AF]/atrial flutter)-free survival in patients with advanced interatrial block (IAB) compared with a similar group of patients with partial IAB in the first article to describe this association. Adapted with permission from Bayés de Luna et al.1 Supraventricular tachyarrhythmia (atrial fibrillation [AF]/atrial flutter)-free survival in patients with advanced interatrial block (IAB) compared with a similar group of patients with partial IAB in the first article to describe this association. Adapted with permission from Bayés de Luna et al.1](https://static.elsevier.es/multimedia/18855857/0000007300000009/v1_202008242214/S1885585720302747/v1_202008242214/en/main.assets/thumbnail/gr4.jpeg?xkr=eyJpdiI6IksxZ21VOGJ6SjBQd0ErSnhmQ3JkWEE9PSIsInZhbHVlIjoiN21JVUNoVDNHSjEvekt3YkJZYzM5YmVkaGlmWTFMdWhaUE9JODFvOW5QVT0iLCJtYWMiOiJhYmYwODE4YjUyOTQ2ODkyNGRjZDVjMzhjMzIwZWJmOGNhYTQzNWNiZmY0M2E0YTcwNTJmMzRlMGM1YjM1YTNlIiwidGFnIjoiIn0=)
