In recent years, robotic-assisted percutaneous coronary interventions (R-PCI) have been used as a feasible, effective, and safe alternative to manual PCI.1 The initial results have been favorable,1 and in the last decade, despite the complexity of robotic procedures increasing their difficulty,2 these good results have persisted,3 with the added benefit of minimized radiation exposure and reduced orthopedic problems derived from the lead apron.4,5
Because this technology continues to be introduced in catheterization laboratories, evidence of day-to-day R-PCI is scarce. Therefore, we present a retrospective registry of the first 58 consecutive R-PCI cases (64 coronary stenosis) performed in a tertiary center between June 2021 and January 2022. All patients had severe coronary artery disease (CAD) with an indication for revascularization due to symptoms, induced ischemia, or previous diagnosis of severe CAD in a high-risk territory (either on coronary computed tomography or a previous angiogram). All procedures were performed with the Robotic CorPath 200 System (Corindus Vascular Robotics, United States), where a bedside sterile cassette engaged to the guiding catheter allows remote control of wires and devices from the control console. All patients signed all pertinent informed consent forms both for tests and publication, and the work was approved by the ethics committee of our center.
All procedures were performed under conscious sedation. The patients were followed-up after the procedure, and events, including death, myocardial infarction (MI), angina, bleeding, stroke, heart failure decompensation, or renal function impairment were recorded.
The median interquartile range age of the patients was 64 [54-77] years and 43 (74.1%) were male. The prevalence of cardiovascular risk factors was high: 67.2% had hypertension, 29.3% diabetes mellitus, 69% dyslipidemia, and body mass index was 27.81 [24.83-31.49] kg/m2; 43 (74.1%) had a prior history of CAD, with a median ejection fraction of 55% [45%-60%].
Twenty patients (34.5%) were symptomatic with induced ischemia on stress imaging. Thirty-eight (65.5%) were staged procedures of non-culprit lesions in patients with a prior MI. Except for 3 cases, all interventions were performed through the radial approach (94.8%), and we treated a total of 64 stenotic lesions localized either in the left anterior descending (31.2%), circumflex (23.4%), right (31.2%), or side coronary branches (14.2%). When simultaneously measuring radiation (microGy/seg) at the usual first operator position and at the control console, exposure was reduced by 98.2%.
Successful robotic revascularization was achieved in 61 (95.3%) lesions. However, manual conversion was required in 6 patients. In 1 patient, we could not cross the lesion due to severe calcification requiring rotational atherectomy; in another patient, the lesion resulted in a chronic occlusion with manual procedure failure; and in another patient, we could not advance the wire because of extreme tortuosity. After stent deployment, manual conversion was required in 1 patient complicated with a coronary perforation treated with microspheres. Two patients had catheter-tip thrombosis and embolization with intraprocedural MI treated with tirofiban and balloon inflation.
There were no immediate in-hospital complications, and the median time to discharge was 5.5 [4.5-8] hours. At a minimum of 30 days of follow-up, there were no major events related to the use of R-PCI. One patient had axillar artery thrombosis 5 days after the procedure, presumably related to manual manipulation of the catheter, and another had an MI in another coronary territory.
Assessment of the main procedural findings according to our learning curve (table 1) revealed that, despite a tendency to more complex lesions (figure 1), in the second half of our R-PCI experience (months 5-8 of training), contrast use and procedural time significantly decreased, with lower rates of fluoroscopy time and total radiation related to better knowledge of the system, and a high success rate, lesser need for manual conversion, and the absence of intraprocedural complications.
Procedural characteristics of overall and first and second half of robotic interventions
| First quarter | Second quarter | P | N | ||
|---|---|---|---|---|---|
| Patients | n=64 | n=31 | n=33 | ||
| AHA lesion: | .776 | 64 | |||
| A | 11 (17.2) | 6 (19.4) | 5 (15.2) | ||
| B1 | 20 (31.2) | 11 (35.5) | 9 (27.3) | ||
| B2 | 20 (31.2) | 8 (25.8) | 12 (36.4) | ||
| C | 13 (20.3) | 6 (19.4) | 7 (21.2) | ||
| Complex lesion | 33 (51.6) | 14 (45.2) | 19 (57.6) | .458 | 64 |
| Number of vessels treated | 1.12 (0.33) | 1.07 (0.26) | 1.17 (0.38) | .235 | 58 |
| Number of stents per patient | 1.00 [1.00-2.00] | 1.00 [1.00-1.00] | 1.00 [1.00-2.00] | .124 | 58 |
| Length of stenting, mm | 21.0 [17.5-32.0] | 20.0 [16.0-28.0] | 23.0 [18.0-34.0] | .178 | 63 |
| Mean diameter, mm | 2.75 [2.62-3.00] | 2.75 [2.62-3.00] | 2.75 [2.62-3.00] | .877 | 63 |
| Contrast, mL | 170 [125-225] | 190 [150-242] | 160 [105-190] | .016 | 58 |
| Fluoroscopy time | 16.0 [11.0-22.9] | 19.0 [15.0-28.0] | 14.5 [9.20-22.0] | .077 | 58 |
| Procedural time, min | 74.0 [53.0-105] | 88.0 [62.0-109] | 64.0 [45.0-79.8] | .011 | 58 |
| PDA, microGy/m2 | 9532 [5155-12 552] | 9532 [4783-11 344] | 9147 [5534-13 834] | .461 | 58 |
| Need to change guiding catheter | 8 (12.5) | 5 (16.7) | 3 (9.38) | .467 | 64 |
| Manual conversion | 6 (9.38) | 5 (16.1) | 1 (3.03) | .099 | 64 |
| Intraprocedural complications | 4 (6.25) | 4 (12.9) | 0 (0.00) | .053 | 64 |
| Successful R-PCI | 61 (95.3) | 29 (93.5) | 32 (97.0) | .607 | 64 |
AHA, American Heart Association; PDA: Product dose area; R-PCI, robotic percutaneous coronary intervention.
Values are shown as No. (%) or median interquartile range.
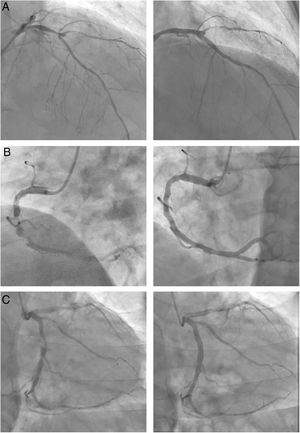
Images on the right show the final results of the 3 revascularizations. A: bifurcated lesion in the mid-left anterior descending artery involving the origin of the diagonal, with an aneurysm between branches. B: chronic suboclusion in the mid-right coronary artery, which was highly calcified, eccentric and diffuse. C: severely eccentric and tubular lesion in the proximal circumflex.
As novices in this technique, we identified the following technical maneuvers that helped us in our daily practice with this robotic modality:
Support is everything. In our cohort, a shift of the guiding catheter was required in 8 (12.5%) patients, 4 of them within the first 30 days of training. Additional support was achieved with a guiding catheter extension (Guideliner, Vascular Solutions, United States), which could be precisely managed as a “device” by the console.
When preparing to cross the guidewire, the assembling of the balloon or the stent over the wire in a single attempt can spare exchange times; this is particularly important, mainly in the early procedures, as it represents a new competence for the nursing team.
During displacement of the guiding catheter from the coronary ostia during PCI, “pulling the device” while maintaining balloon inflation, as can be done advancing the catheter during manual PCI, can mostly solve inadequate coronary engagement.
After stent deployment, pushing forward the device (stent balloon) to ensure its complete release before its withdrawal can help to avoid deep catheter intubation.
Use high doses of heparin. Catheter tip thrombus occurred in 2 patients during the first trimester of training who received a weight-adjusted heparin dose (100 UI/kg). However, since we systematically administered 10 000 UI, no intracoronary thrombus has been documented and no bleeding complications have appeared so far.
In conclusion, prior evidence indicates that R-PCI is safe and effective,1,2 and opens new horizons in the field of coronary revascularization. Evidence on its ability to perform complex PCI—including AHA C lesions, chronic total occlusions and left main disease—has been reported, maintaining outstanding clinical and procedural results,3,5 and real-life data of this robotic technology in daily PCI is currently ongoing. Our early experience is favorable, although limited by selection bias, as it was gained in a single center and we lacked a control cohort to match our findings. Available models so far have certain limitations, as they allow for manipulation of only 1 coronary guidewire or device at a time, are incompatible with intracoronary imaging techniques, and have high costs. These and other technical limitations can be addressed through iteration and innovation.6 Nevertheless, early experience requires adequate lesion selection, with cumulative experience procedural times tend to shorten, and better skills are acquired to deal with higher levels of complexity (figure 1).
FUNDINGM. Tamargo is supported by grant Rio Hortega, CM20/00054 from the Instituto de Salud Carlos III, Spain.
AUTHORS’ CONTRIBUTIONSAll authors meet each and all the characteristics defined by the International Committee of Medical Journal Editors for the criteria for authorship of scientific articles.
CONFLICTS OF INTERESTNothing to disclose.