More than 2 decades ago, the approach to coma patients who had recovered from out-of-hospital cardiac arrest (OHCA) basically consisted of offering life support measures and waiting for spontaneous neurological recovery.1 Such improvement was rare and only in this instance was an etiological study of the underlying heart disease initiated. With the advent of therapeutic hypothermia, the perception arose that an active approach might be needed to facilitate patient recovery and prevent or minimize the consequences of the phenomenon known as postcardiac arrest syndrome,2 which is due to massive body damage caused by prolonged ischemia followed by reperfusion. This change in attitude coincided with the demonstration in the DANAMI-23 study of the benefit of transferring patients with ST-segment elevation acute coronary syndrome (STEMI) to hospitals with a permanent capacity for coronary intervention, even if they were not the closest ones. Until then, reperfusion was mainly performed using systemic fibrinolysis, although OHCA settings are not well suited to this approach, particularly after prolonged resuscitation periods due to possible trauma during resuscitation maneuvers. Therefore, pharmacological reperfusion was only offered to a few patients after short-term resuscitation.
Over time, the idea began to take hold that patients who had recovered from OHCA should probably also be transferred to hospitals with a permanent capacity for coronary intervention, if needed. This strategy was recommended in clinical practice guidelines, since ischemic heart disease is the main cause of CHD in the adult population.4 This idea was supported by registry data that suggested that patients treated in hospitals with the capacity for coronary intervention at any time had better outcomes.5 However, it was evident that there was selection bias following confirmation that patients selected for early coronary intervention had a more favorable risk profile or indisputable evidence of STEMI.6 This was the seed that indicated the need to study the possible role of systematic and early coronary angiography in resuscitated OHCA patients.
The article by Viana-Tejedor et al., recently published in Revista Española de Cardiología, presents the results of the randomized COUPE clinical trial.7 It is the sixth such article to be published and attempts to shed some light on this still unresolved issue.7–12 The COUPE trial, like previous trials, did not demonstrate the superiority of an initial invasive or conservative strategy for comatose patients who have recovered from non-STEMI OHCA. The information obtained from the COUPE trial and previous studies, however, provides food for thought regarding the clinical trials conducted on this condition, which may prove useful in the design and execution of future studies.
DESIGN OF STUDIES ON THE ROLE OF ANGIOGRAPHY AFTER RESUSCITATIONIn general, and to a greater or lesser degree, the studies were designed7–12 according to a pragmatic approach using few exclusion criteria, thereby seeking to generalize the results by including patients without age limits, in any initial rhythm, with comorbidities, and so on. Usually this could be a virtue in itself, since the use of pragmatic studies is the preferred approach for defining a diagnostic-therapeutic strategy, although they may not be suitable if the sample size is not adapted to the differences in prognosis that may be caused by randomizing treatment to populations with heterogeneous risks. In terms of risk factors, the fact that there may not be significant differences between experimental treatment groups and control groups does not mean that the risks are equal, especially if the sample is small. The complexity of the situation after OHCA means that, in all of the studies, the projected sample size was insufficient to compensate for the innumerable confounding factors that are related to prognosis and were not considered in the distribution of the study arms. If studies are not sufficiently sized, chance could tip the balance to one side or the other. Factors such as the patients’ comorbidities, initial rhythm, duration of resuscitation, its quality and etiology, among many others, can only be compensated for by stratified randomization or large sample sizes.
On the other hand, the choice of the primary endpoint under study is also relevant. With one exception, the studies selected all-cause mortality (ARREST8, COACT,11 and TOMAHAWK12) or survival with good neurological outcome (PEARL10 and COUPE7) as the primary endpoint. However, the DISCO9 pilot study, which was designed to explore the feasibility of a clinical trial in this setting, only provided information on 24-hour mortality. However, if a strategy of systematic and early angiography is expected to reduce OHCA mortality, such reductions would be due to decreases in cardiovascular mortality in these patients because of their treatment for ischemic heart disease, rather than because of a reduction in other causes of death. None of the 6 studies cited took into account that the main cause of death in patients in post-OHCA coma is the limited therapeutic effort due to predictable poor neurological outcomes. This situation is the cause of death in 73% of such patients, whereas cardiovascular emergencies, such as cardiogenic shock or new cardiac arrest, are the cause in only 21% of these patients. In a smaller percentage of patients, the cause is limited therapeutic effort due to other comorbidities or conditions.13 Therefore, to significantly reduce all-cause mortality, an exclusive reduction in cardiovascular mortality—which is what could be expected from early revascularization—would require a much larger sample than those used in the 6 clinical trials. The same would be true for survival with good neurological outcome, for which there is little pathophysiological basis to argue that early angiography could have a large positive impact other than that due to reduced cardiovascular mortality. Therefore, cardiovascular mortality could be a less ambitious primary outcome.
In the world of cardiology, this approach is well known and accepted in other settings, given the difficulty in demonstrating benefits by the use of “hard” targets such as all-cause mortality and in achieving the huge sample size that would be required. In heart failure or ischemic heart disease, for example, it is not uncommon for the primary outcome of a study using a novel treatment to be able to demonstrate its effect on cardiovascular mortality and rehospitalization.14 The recently published TTM-2 trial15 may also serve as an illustration. This study was designed to assess the effect of temperature management at the targets of 33°C or <37.8°C using a primary outcome of all-cause mortality. The expected effect of temperature management was to decrease neurological damage and thereby achieve lower total mortality. During the 6-month study period, 911 patients died out of the 1850 patients analyzed. The study showed no benefit from the use of either of the temperature thresholds. However, the cause of death in one-third of the patients who died was the limited therapeutic effort during the first few days of the study, and this was not because of an expected poor neurological outcome, but because the patients had serious comorbidities. This eventuality significantly reduced the statistical power of the study. Perhaps a more reasonable design would have used neurological mortality or severe neurological sequelae. These options have never been raised in the setting of critically ill patients and could be considered when designing future studies, especially in situations in which multiple factors may have an effect on prognosis.
Therefore, if it is not possible to realistically size an ambitious and definitive target, such as all-cause mortality, it is probably more appropriate to size it for a more humble target that is capable of resolving some of the unknowns we face.
SELECTIVE ANGIOGRAPHY OR SYSTEMATIC ANGIOGRAPHY FOR ALL PATIENTS RECOVERED FROM OHCAUnfortunately, the COUPE study could not be completed due to a very slow patient inclusion process that did not reach the sample size projected at the beginning of the study. The question remains as to what the results might have been if patient inclusion had been completed and if it would have been useful in relation to performing early angiography or not in all patients. Although the authors of the COUPE trial attribute the low inclusion rate largely to the impact of the COVID-19 pandemic, which led to a reduction in the number of patients recovering from OHCA, it can be inferred that the pandemic had little impact on the study. The study began in 2016 and was expected to conclude in 3 years. However, the patient inclusion rate was lower than expected over the study period, and was only affected by the pandemic in the last 10 months of the nearly 5-year patient inclusion period. The pandemic may have influenced the study's premature termination, in the sense that it was probably assumed that its completion would have been excessively delayed. The most likely cause of the low patient inclusion rate is the enormous difficulty in conducting clinical trials in critically ill patients, especially if they are patients with high mortality rates, since the investigator and the patient's relatives may think that the patient could be deprived of the possible benefit of an action that is thought to be useful, rather than leaving to chance the choice of treatment that could deprive the patient of this action. For this reason, the authors of the COUPE trial should be congratulated for attempting to conduct it and publish the results, even though they may have been left with a bitter taste when they decided to discontinue it prematurely.
At the present time, an early invasive approach is indisputable and its usefulness has never been questioned for recovered OHCA patients who show ST-segment elevation on the electrocardiogram after heartbeat recovery, patients with hemodynamic instability, or patients with recurrent malignant ventricular arrhythmias with high suspicion of myocardial ischemia. This approach is also recommended for patients who have not experienced OHCA. Therefore, there is every reason to believe that it should also be recommended for patients who have undergone OHCA, unless the percentage of patients with a poor prognosis is higher. However, questions remain regarding patients not meeting these characteristics.
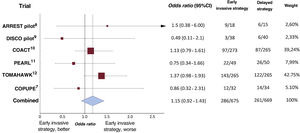
Based on the findings of the 6 clinical trials performed to date, and pending publication of those still in progress (figure 1), a systemic invasive approach is not justified in patients with hemodynamic and electrical stability. Even in the TOMAHAWK trial,12 143 of 265 patients (54.0%) in the immediate angiography group and 122 of 265 patients (46.0%) in the delayed angiography group had died at 30 days (hazard ratio, 1.28; 95% confidence interval, 1.00-1.63; P=.06). This phenomenon can be attributed in part to the greater neurological deterioration observed in the invasive strategy group, which could be explained by the delayed use of other strategies with a more well-founded beneficial effect in the treatment of these patients, such as temperature control, which has been confirmed in several clinical trials. The approach of performing all diagnostic tests as soon as possible to obtain very early prognostic information, even if they do not have a proven impact on the patients’ course, should not delay treatments with some scientific evidence of efficacy. This is one of the reasons offered for the lack of efficacy in some recent clinical trials on temperature control. The efficacy of this type of treatment had been demonstrated before systematic angiography and other very early diagnostic tests became widespread, which was partly motivated by clinical practice guidelines.4 The aforementioned TTM-2 trial is of particular interest because it is the clinical trial in which the earliest angiograms were performed15; most patients underwent coronary angiography (78%), almost all of which were performed within the first 2hours of admission (90%), and less than half of the patients were revascularized (39%). A brain computed tomography scan was also performed in 67% of the patients, generally at the time of admission.
Forest plot of short-term all-cause mortality using data obtained from randomized studies on the effect of routine early angiography in comatose patients recovered from out-of-hospital cardiac arrest without ST-segment elevation on electrocardiogram after recovery of spontaneous circulation. 95%CI, 95% confidence interval.
In daily clinical practice, the appropriate approach is probably to use an early invasive strategy in patients with ST-segment elevation and/or electrical or hemodynamic instability. However, in other situations, and given the currently available information, the best approach would be to immediately initiate therapeutic measures with proven efficacy in at least 1 clinical trial, and to reserve diagnostic tests for a later time if the need arises or after neurological recovery has been confirmed.16 In addition, decisions on the withdrawal of life-support measures should be considered after sufficient time has elapsed in order to avoid possible errors.
FUNDINGNone declared.
CONFLICTS OF INTERESTNone declared.