The epidemiology, microbiology, and diagnosis of infective endocarditis (IE) has changed rapidly over the last 50 years. The International Collaboration on Endocarditis (ICE) study1 reported that the most prevalent causes of IE were degenerative valve disease and the presence of prosthetic valves (in the 1960s and 1970s the main causes were rheumatic lesions). Moreover, the very recent EURO-ENDO observational study has highlighted a change in the microbiology of IE—the most prevalent causative microorganisms are staphylococci (44%), followed by enterococci (15.8%), oral streptococci (12.3%), and Streptococcus gallolyticus (6.6%)—and enterococci are no longer the third cause of IE, as they were at the time of the ICE study, and have become the second cause. This is probably due to the increasing proportion of older patients with comorbidities affected by IE, which also makes IE a disease with poor prognosis.2
Gut permeability and inflammation, being more prevalent in the older population, could explain gut bacteria “leakage” in the systemic circulation, potentially causing not only cardiovascular but also autoimmune diseases.3 Enterococci are the most abundant bacteria in the human gastrointestinal tract (small and large intestine), representing 1% of the human fecal flora, with Enterococcus faecalis and Enterococcus faecium being the 2 main species of the enterococcus genus. In healthy individuals, enterococci play a commensal/probiotic role but, in elderly people, who are more frequently affected by dysbiosis (an imbalance in the community of healthy human gut microbiota), bacteria can cross the mucosa of the gut and enter the circulation. One of the many ways the microbiota maintain intestinal homeostasis is by stimulating goblet cell secretion of mucin, which is a thick layer protecting the colonic epithelium from intestinal microbes. Whenever this commensal role is not achieved, bacteria can enter the circulation and colonize other areas of the body and form biofilms.4 Chronic dysbiosis associated with other factors, such as genetics and diet-derived chemical irritants, can induce chronic colonic inflammation and lead to colorectal diseases and colorectal neoplasia (CRN), which can progress to colorectal cancer (CRC).5
Enterococci are not the only bacteria able to cross the mucosa of the colon, S. gallolyticus, which is, however, not present in most human gut (2.5%-15% of healthy humans), also has this property. S. gallolyticus bacteremia has been extensively studied and the mechanism of translocation and dissemination is well understood. Translocation happens through surface-exposed adhesins allowing adhesion to host cells, whereas dissemination involves the pilus (Pil3 expression), which binds to plasma fibrinogen. Once in the blood stream, Pil1 expression is responsible for the binding to collagen I (present on the surfaces of damaged heart valves), achieving heart colonization.6 As long ago as the 1970s, S. gallolyticus bacteremia was found to be associated with both CRN and IE.7
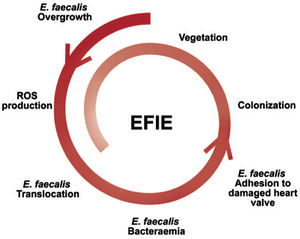
In contrast, the mechanism of E. faecalis bacteremia is less well known. Although the main portal of entry of E. faecalis is the genitourinary tract, it has been shown that E. faecalis overgrowth can stimulate reactive oxygen species production and induce genomic instability in colonic cells, favoring lesions of the intestinal mucosa and translocation of E. faecalis in the blood circulation8 (figure 1).
Supporting the hypothesis of the gastroenteric tract as a portal of entry of E. faecalis bacteremia, a study published in 2017 by Pericàs et al. explored the relationship between E. faecalis IE (EFIE) and CRN. These authors examined the prevalence of colorectal neoplasms in EFIE patients whose presumed source of infection could not be determined and found that 50.8% had CRN. In the group of patients with an identified portal of entry of the infection, only 6 patients underwent colonoscopy and 1 of them was diagnosed with CRN (16.7%); therefore no conclusions could be drawn for this group.9
In an article by Escolà-Vergé et al.,10 recently published in Revista Española de Cardiología, the authors aimed to determine the prevalence of colorectal disease in EFIE patients, irrespective of the hypothesized source of infection. The rationale behind their study was based on the fact that 5% of patients with IE (all etiologies) would experience recurrent infection with a higher mortality risk, the reason being a potential undiagnosed colorectal disease (portal of entry of bacteria into the circulation). If this hypothesis is correct, performing a colonoscopy, which could identify and treat the upstream cause of the endocarditis, would decrease the risk of recurrence. In their study, the authors included all consecutive episodes (n = 103) with EFIE seen in 4 referral centers (3 centers in Spain and 1 in Italy). Patients were classified based on the presumed source of infection into patients with “unknown portal of entry” and patients with “known portal of entry”. Only 70 EFIE patients underwent colonoscopy (6 months before or after the EFIE diagnosis): patients with an “unknown portal of entry” were more numerous than those with an “known portal of entry” (64% vs 36%).
Comparison of demographic and clinical characteristics between the 2 groups revealed that health care-associated infections were more prevalent in patients with a “known portal of entry” than in those with an “unknown portal of entry” (83% vs 29%, P < .001). The reason for this difference is probably that patients with a presumed source of infection were more frequently affected by chronic renal failure than patients with an unknown source of infection (30% vs 21%). In addition, patients with a presumed source of infection had more frequently undergone immunosuppressive treatment and liver transplant (15% vs 6% and 15% vs 3%).
Within the subgroup with an “unknown portal of entry”, the authors noted that 64% of patients had endoscopic findings, while within the subgroup with an “known portal of entry” (different from gastrointestinal origin) 44% showed endoscopic findings.
As expected, among EFIE patients with a presumed gastrointestinal source of infection, 88% of them had a colorectal disease diagnosis after colonoscopy and, surprisingly, 44% patients with a presumed urinary source of infection had endoscopic findings. In these patients, the question remains whether the portal of entry is the colon or the urinary tract. Assuming that the portal of entry is the urinary tract, the high percentage of endoscopic findings in this group can be explained by the fairly high prevalence of colorectal diseases in this age group.11
The result of the study is relevant since, in total, 60% of the EFIE patients who underwent colonoscopy (including patients with a presumed gastrointestinal source of infection) had endoscopic findings. Based on histopathological characteristics, the colorectal findings identified were classified into neoplastic and nonneoplastic. Theoretically, each colonic lesion found could represent a portal of entry (uncomplicated diverticula and hemorrhoids were not included). Most endoscopic findings were neoplastic, 83% were advanced and nonadvanced colorectal adenoma, and 17% were nonneoplastic. Among the EFIE patients, 50% of them had neoplastic colorectal disease, which is a higher percentage than the percentage of colorectal adenoma in the general elderly population (33% in the Greek asymptomatic population aged > 50 years11). There was only 1 case of CRC in the subgroup of patients with an unknown source of infection and no cases of CRC in patients with a known source of infection. Escolà-Vergé et al. also looked at differences between the groups “no endoscopic findings” vs “endoscopic findings” to see if the group without endoscopic findings was younger. There were no statistically significant age differences between the 2 groups. However, the group with endoscopic findings had more comorbidities (diabetes mellitus and chronic renal failure). Moreover, there were more male patients in the “endoscopic findings” group than in the “no endoscopic findings” group (81% vs 77%). The 4% difference, which was not statistically significant, could be due to the fact that men are at greater age-specific risk for colorectal diseases than women12,13 and that urinary infections are the main portal of entry for women in this population (the percentage of patients with a presumed urinary source of infection was higher in the “no endoscopic findings” group than in the “endoscopic findings” group (29% vs 15%). This would be suggested by the fact that women are at greater risk for urinary tract infections.14
A limitation of the study is that it has a retrospective design and a small sample size. In addition, 80% of the EFIE patients who underwent colonoscopy were male, meaning that there were only 16 female patients who underwent colonoscopy: 7 with no findings (44%) and 9 with findings (56%). It is understandable that a subgroup of EFIE patients extrapolated from an IE population would include more men, since the prevalence of IE in men is higher compared with age-matched women (in the EURO-ENDO study only 30% are women)2 and since colorectal diseases are more common in men than in women 13 (colorectal diseases being the main cause of EFIE).
It would have been interesting to know the presumed source of infection in the 9 female patients, which, unfortunately, cannot be deduced from the demographic features listed in the tables of the article. The authors eventually had to conduct the study considering female and male patients separately. Several studies have speculated that the characteristics of IE differ between men and women.15
The authors also determined relapses in all the EFIE patients with a minimum follow-up of 3 months. There were only 2 relapses and both occurred in the group of patients with relevant endoscopic findings. The percentage of relapses in colonoscopy-screened patients would therefore be almost 50% lower than in the general population (from 5% to 2.8% relapse: 2 in 70 cases).
In conclusion, EFIE is a good predictor of colorectal disease. Treating colorectal conditions could avoid IE recurrence. In our opinion, this study could help to prompt larger studies, which may result in proposals for changes in the next EFIE guidelines. Such changes would benefit patients with EFIE, since they would be diagnosed and treated earlier for colorectal disease, which could not only avoid malignant transformation of the colorectal condition but also hinder endocarditis recurrence. Further clinical studies should be performed to lower the frequency of colonic enterococci and gastrointestinal portal of entry, which is now at 15.8%, as reported in the EURO-ENDO study.2
CONFLICTS OF INTERESTThe authors have no conflicts of interest to disclose.
This work was supported by a ERC-Consolidator grant (Project Number: 647197). C. Oury is Senior Research Associate at the National Funds for Scientific Research (F.R.S.-FNRS, Belgium). We also want to thank Alexandre Hego for having improved the quality of the image depicted in figure 1.