To assess the cost-effectiveness of edoxaban vs acenocoumarol in the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (NVAF) in Spain.
MethodsMarkov model, adapted to the Spanish setting from the perspective of the National Health System, stimulating the progression of a hypothetical cohort of patients with NVAF throughout their lifetime, with different health states: stroke, haemorrhage, and other cardiovascular complications. Efficacy and safety data were obtained from the available clinical evidence (mainly from the phase III ENGAGE AF-TIMI 48 study). The costs of managing NVAF and its complications were obtained from Spanish sources.
ResultsEdoxaban use led to 0.34 additional quality-adjusted life years (QALY) compared with acenocoumarol. The incremental cost with edoxaban was 3916€, mainly because of higher pharmacological costs, which were partially offset by lower costs of treatment monitoring and managing NVAF events and complications. The cost per QALY was 11 518€, within the thresholds commonly considered cost-effective in Spain (25 000-30 000 €/QALY). The robustness of the results was confirmed by various sensitivity analyses.
ConclusionsEdoxaban is a cost-effective alternative to acenocoumarol in the prevention of stroke and systemic embolism in patients with NVAF in Spain.
Keywords
Atrial fibrillation (AF), with an estimated prevalence of 3%, is the most common arrythmia in the western world.1 It affects men and women equally and becomes more common with age; in Spain, 4.4% of patients with AF are older than 40 years. In addition, its prevalence increases in a stepwise fashion after the age of 60 years (17.7% of patients are older than 80 years).2 Over 1 million people are estimated to have AF in Spain and approximately 90 000 of these are undiagnosed.2
AF is the main cause of embolic events, of which the most common and most serious is stroke. Patients with AF are about 5 times as likely as those without AF to have a stroke,3 and they also have a higher risk of disability, recurrence, and mortality.4 The incidence of stroke in patients with AF not receiving anticoagulants is 3.1 cases per 100 patient-years vs 0.3 cases in those receiving anticoagulants.4
Stroke is the first cause of adult disability worldwide, the second cause of dementia, and the third cause of death. In patients older than 75 years, it is the main cause of death. It is also one of the most costly diseases from a socioeconomic perspective5,6 due to hospitalization and informal care costs.7 Nevertheless, many of the events associated with a diagnosis of AF can be prevented with anticoagulant therapy.8
Nonvalvular AF (NVAF) occurs in patients without a mechanical heart valve and without moderate to severe mitral stenosis (generally of rheumatic origin).9 The standard prophylactic treatment for stroke is oral anticoagulant therapy with vitamin K antagonists (VKAs) or direct oral anticoagulants (DOACs).10 Although warfarin is the mainstay of VKA therapy, acenocumarol is more widely used in Spain.4 The procedures needed to check international normalized ratio (INR) values and monitor hemorrhagic and/or thromboembolic risk are similar for warfarin and acenocoumarol.11 Although VKAs are an effective treatment, they have several disadvantages that complicate their use. They have high dose-response variability, which requires regular INR testing and dose adjustments to ensure that the treatment has the desired effect but does not cause serious adverse outcomes, such as stroke or hemorrhage.12 Inadequate INR control determined using the direct method is associated with a time in therapeutic range of less than 60%10 (40%-54% of patients in Spain).13 In addition, the need for frequent testing and the risk of potentially harmful interactions with other drugs have traditionally been associated with underuse of VKA therapy in AF.14 Moreover, reports of adverse reactions to VKAs have increased.15 Adherence to VKA therapy in Spain is just 50%4 and there is thus an increased risk of stroke and hemorrhage.12
DOACs have the necessary efficacy and safety profile to meet most needs not covered by VKAs16,17: they have a wide therapeutic window, a predictable anticoagulant response unaffected by food intake, and few interactions with other drugs. In addition, they offer a constant anticoagulant effect and do not require regular monitoring or repeated dose adjustments.12
High-dose edoxaban is the most recent DOAC to be authorized for the treatment of NVAF in Spain. It is administered as a single daily dose of 60mg or, in the following cases, 30mg: moderate to severe kidney failure (creatinine clearance, 15-50mL/min), low body weight (≤60kg), and concomitant use of certain glycoprotein inhibitors (ciclosporin, dronedarone, erythromycin, and ketoconazole). Edoxaban is a direct oral selective factor Xa inhibitor. Based on the results of the ENGAGE AF-TIMI 48 clinical trial, high-dose edoxaban is at least as efficacious as warfarin in preventing stroke and systemic embolism and it is significantly safer than well-controlled warfarin therapy in terms of major bleeding events.18,19
The aim of this study, conducted in Spain, was to evaluate the cost-effectiveness of edoxaban in preventing stroke and systemic embolism in adults with NVAF and 1 or more risk factors.
METHODSStudy Population and ComparatorsThe characteristics of the population used for the base-case analysis were the same as in the ENGAGE AF-TIMI 48 trial,19 namely, a diagnosis of NVAF, a moderate to high risk of stroke (CHADS2 stroke risk score ≥ 2), a higher proportion of men than women (62.29%), and a mean age of 71 years.
We also compared high-dose edoxaban with VKA therapy,19 but instead of warfarin we analyzed acenocoumarol, the most widely used VKA in Spain,4 and assumed that the 2 drugs had identical safety and efficacy.
Type of AnalysisWe estimated the mean per-patient cost-effectiveness of each treatment, calculated the incremental cost-effectiveness ratio of edoxaban vs VKA therapy, and determined the additional cost required to gain 1 quality-adjusted life year (QALY) with edoxaban.
The costs were analyzed from the perspective of the Spanish national health system and included direct medical costs (calculated in 2017 euros). The time horizon contemplated in the analyses was the patient's full lifetime. An annual discount of 3% was applied for costs and future effects.20
A group of experts selected for their experience and familiarity with clinical practice was formed to help adapt the necessary aspects of the study to Spain. The members of this group filled in individual questionnaires and were then presented with a base case for evaluation.
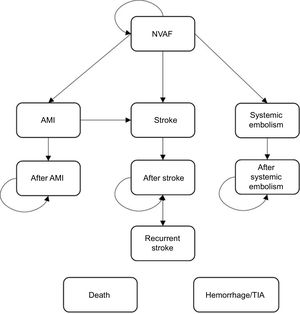
Pharmcoeconomic ModelA Markov model was used to simulate the course of events in patients with NVAF and a moderate to high risk of stroke through the analysis of different clinical situations (health states) associated with the use of different resources and specific clinical outcomes. A simplified version of this model is shown in Figure 1. The transition between health states and the resulting clinical/economic outcomes were evaluated in monthly cycles. The probability of a patient progressing from a milder to a more serious state, depending on the case, was derived from the clinical outcomes of the treatments.
At the start of the analysis, the patients had a stable NVAF status, and during each cycle they transitioned between the following health states: hemorrhagic or ischemic stroke (mild, moderate, or severe), systemic embolism, and acute myocardial infarction. These events were associated with an initial impact (acute event) and a long-term impact (postevent)
Other events (complications) considered were bleeding (intracranial, major extracranial, and clinically relevant nonmajor bleeding) and transient ischemic attacks. These were possible in any of the health states.
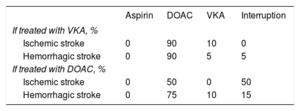
A stroke could be followed by new events (recurrence), complications, and temporary or permanent interruption of treatment (with a treatment switch).
Risk of death varied according to the demographic characteristics of each patient (age and sex) and their health status.
Clinical DataThe clinical data for the model were mainly obtained from the phase III ENGAGE AF-TIMI 48 trial19 and available scientific evidence.
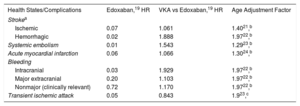
The monthly transition probabilities are shown in Table 1. It was assumed that the risk of an event increased with age.
Monthly Transition Probabilities and Age Adjustment Factors
| Health States/Complications | Edoxaban,19 HR | VKA vs Edoxaban,19 HR | Age Adjustment Factor |
|---|---|---|---|
| Strokea | |||
| Ischemic | 0.07 | 1.061 | 1.4021,b |
| Hemorrhagic | 0.02 | 1.888 | 1.9722,b |
| Systemic embolism | 0.01 | 1.543 | 1.2923,b |
| Acute myocardial infarction | 0.06 | 1.066 | 1.3024,b |
| Bleeding | |||
| Intracranial | 0.03 | 1.929 | 1.9722,b |
| Major extracranial | 0.20 | 1.103 | 1.9722,b |
| Nonmajor (clinically relevant) | 0.72 | 1.170 | 1.9722,b |
| Transient ischemic attack | 0.05 | 0.843 | 1.923,c |
VKA, vitamin K antagonist; HR, hazard ratio.
The monthly risk of stroke recurrence is 0.25% for ischemic stroke and 0.26% for hemorrhagic stroke.25 This risk increases to 0.44% following an acute myocardial infarction.25
Data on treatment interruption and switching following a stroke were based on the opinions of the expert group (Table 2).
Three types of mortality were considered for the analysis: overall mortality26 (adjusted for the risk associated with AF, hazard ratio [HR] = 1.3427), mortality due to an acute event, and mortality after surviving an acute event (Table 3).19,28–32
Probability of Mortality Due to an Acute Event and After Surviving an Acute Event
| Probability of mortality due to an acute event | ||
|---|---|---|
| Event | Probability (%) | Source |
| Ischemic stroke | ||
| Mild | 0.0 | Assumption |
| Moderate | 16.8 | Janes et al.28 |
| Severe | 16.8 | Janes et al.28 |
| Hemorrhagic stroke | ||
| Mild | 0.0 | Assumption |
| Moderate | 31.6 | Janes et al.28 |
| Severe | 31.6 | Janes et al.28 |
| Systemic embolism | 0.0 | Giugliano et al.19 |
| Acute myocardial infarction | 13.2 | Scarborough et al.29 |
| Intracranial bleeding | 31.6 | Janes et al.28 |
| Major nonintracranial bleeding | 0.0 | Assumption |
| Nonmajor bleeding (clinically relevant) | 0.0 | Assumption |
| Transient ischemic attack | 0.0 | Assumption |
| Probability of mortality after surviving an acute event | ||
|---|---|---|
| Previous event | HR* | |
| Ischemic/hemorrhagic stroke | ||
| Mild | 3.18 | Bronnum-Hansen et al.30 |
| Moderate | 5.84 | Henriksson et al.31 |
| Severe | 15.75 | Huybrechts et al.32 |
| Systemic embolism | 5.45 | Giugliano et al.19 |
| Acute myocardial infarction | 3.36 | Bronnum-Hansen et al.30 |
HR, hazard ratio.
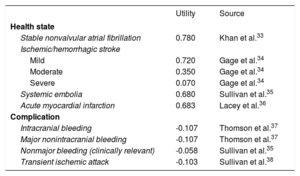
Quality-of-life utility scores in relation to health states fell on a scale of 0 (death) to 1 (perfect health). The utilities for each state and the loss of utility due to complications were taken from the literature (Table 4).33–38
Utilities Used in Model
| Utility | Source | |
|---|---|---|
| Health state | ||
| Stable nonvalvular atrial fibrillation | 0.780 | Khan et al.33 |
| Ischemic/hemorrhagic stroke | ||
| Mild | 0.720 | Gage et al.34 |
| Moderate | 0.350 | Gage et al.34 |
| Severe | 0.070 | Gage et al.34 |
| Systemic embolia | 0.680 | Sullivan et al.35 |
| Acute myocardial infarction | 0.683 | Lacey et al.36 |
| Complication | ||
| Intracranial bleeding | -0.107 | Thomson et al.37 |
| Major nonintracranial bleeding | -0.107 | Thomson et al.37 |
| Nonmajor bleeding (clinically relevant) | -0.058 | Sullivan et al.35 |
| Transient ischemic attack | -0.103 | Sullivan et al.38 |
Despite having a stable INR, patients being treated with a VKA may experience a slight deterioration in quality of life due to anxiety about remaining within the therapeutic range and potential interactions between the drug and other medication or food.39 For the base case, we considered a loss of utility of 0.012 in these patients.40
Use of Resources and CostsThe direct medical costs considered were pharmacologic costs, VKA monitoring costs, and the treatment costs for each event.
Daily pharmacologic costs were calculated according to the established treatment regimens and retail prices,41 with application of the corresponding deduction.42 The resulting estimates were a daily cost of €2.58 for edoxaban (60mg/d) and €0.07 for acenocoumarol (2.75mg/d).
VKA therapy requires regular INR monitoring (14 tests a year43) and possible dose adjustments. According to the expert group, 50% of these tests would be performed in an in-hospital setting and 50% in an outpatient setting. The estimated costs were €20.15 and €30.89, respectively.44 The annual cost of INR monitoring was therefore set at €357.28.
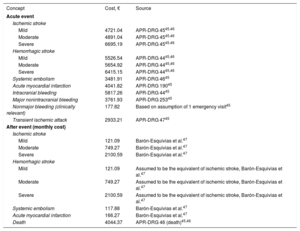
The costs per event type were calculated using data from the relevant literature and opinions expressed by the expert group (Table 5).
Costs Associated With Health States in the Model
| Concept | Cost, € | Source |
|---|---|---|
| Acute event | ||
| Ischemic stroke | ||
| Mild | 4721.04 | APR-DRG 4545,46 |
| Moderate | 4891.04 | APR-DRG 4545,46 |
| Severe | 6695.19 | APR-DRG 4545,46 |
| Hemorrhagic stroke | ||
| Mild | 5526.54 | APR-DRG 4445,46 |
| Moderate | 5654.92 | APR-DRG 4445,46 |
| Severe | 6415.15 | APR-DRG 4445,46 |
| Systemic embolism | 3481.91 | APR-DRG 4645 |
| Acute myocardial infarction | 4041.82 | APR-DRG 19045 |
| Intracranial bleeding | 5817.26 | APR-DRG 4445 |
| Major nonintracranial bleeding | 3761.93 | APR-DRG 25345 |
| Nonmajor bleeding (clinically relevant) | 177.82 | Based on assumption of 1 emergency visit45 |
| Transient ischemic attack | 2933.21 | APR-DRG 4745 |
| After event (monthly cost) | ||
| Ischemic stroke | ||
| Mild | 121.09 | Barón-Esquivias et al.47 |
| Moderate | 749.27 | Barón-Esquivias et al.47 |
| Severe | 2100.59 | Barón-Esquivias et al.47 |
| Hemorrhagic stroke | ||
| Mild | 121.09 | Assumed to be the equivalent of ischemic stroke, Barón-Esquivias et al.47 |
| Moderate | 749.27 | Assumed to be the equivalent of ischemic stroke, Barón-Esquivias et al.47 |
| Severe | 2100.59 | Assumed to be the equivalent of ischemic stroke, Barón-Esquivias et al.47 |
| Systemic embolism | 117.88 | Barón-Esquivias et al.47 |
| Acute myocardial infarction | 166.27 | Barón-Esquivias et al.47 |
| Death | 4044.37 | APR-DRG 46 (death)45,46 |
APR-DRG, All Patient Refined–Diagnosis Related Group.
To characterize the uncertainty surrounding the study input parameters and their potential influence on outcomes, we performed a probabilistic sensitivity analysis using second-order Monte Carlo simulation with a hypothetical cohort of 1000 patients.20 The main parameters from the base-case analysis were varied simultaneously and assigned the following distributions: normal distribution for age of onset; Dirichlet distribution for stroke severity; beta distribution for mortality due to an acute event, utilities, and probabilities (of events and recurrences); log-normal distribution for HRs, mortality following an event, adjustment factors for overall mortality, and risk for each decade of life; and gamma distribution for costs.
The results of the analysis were plotted on a cost-effectiveness plane, where each point corresponded to 1 simulation run according to the stipulated parameters and distributions. The results were also plotted on an acceptability curve.
Additional sensitivity analyses were conducted for the following scenarios:
- •
To account for regional variability at INR testing sites, a scenario where 20% of tests are performed in a hospital and 80% in an outpatient setting and vice versa.
- •
To account for possible overestimation of annual INR testing costs due to the use of potentially outdated data, a scenario contemplating a third of the annual base cost (€119.09).
- •
A scenario with patients with CHADS2 ≥ 3.48.
- •
A scenario with patients with a time in therapeutic range ≥ 60%.48
- •
A scenario in which patients on VKA therapy had no loss of utility.
Time horizons and hence treatment durations are important considerations when evaluating treatment effectiveness. We therefore evaluated different time horizons for both the base case and the scenarios involving the subgroups of patients with a CHADS2 score of at least 3 and time in the therapeutic range of 60% or more.
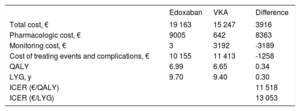
RESULTSBase CaseEdoxaban was more effective than the VKA (0.34 QALYs and 0.30 life years gained) due to lower rates of stroke, intracranial and extracranial bleeding, systemic embolism, and acute myocardial infarction (Table 6).
Base-Case Results
| Edoxaban | VKA | Difference | |
|---|---|---|---|
| Total cost, € | 19 163 | 15 247 | 3916 |
| Pharmacologic cost, € | 9005 | 642 | 8363 |
| Monitoring cost, € | 3 | 3192 | -3189 |
| Cost of treating events and complications, € | 10 155 | 11 413 | -1258 |
| QALY | 6.99 | 6.65 | 0.34 |
| LYG, y | 9.70 | 9.40 | 0.30 |
| ICER (€/QALY) | 11 518 | ||
| ICER (€/LYG) | 13 053 |
ICER, incremental cost-effectiveness ratio; LYG, life years gained; QALY, quality-adjusted life years; VKA, vitamin K antagonist.
Edoxaban was associated with an additional cost of €3916 compared with the VKA. This extra cost was mainly due to the higher price of the drug, but was partially offset by the lower costs of INR monitoring and treatment of events and complications (which are less common with edoxaban) (Table 6). Compared with the VKA, edoxaban was associated with an incremental cost-effectiveness ratio of €11 518 for each QALY gained, which is under the generally accepted threshold for cost-effectiveness (€25 000-€30 000 per QALY).49,50
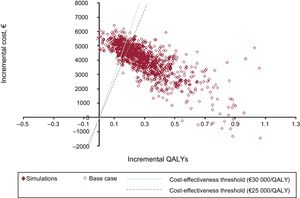
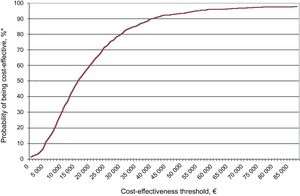
Sensitivity AnalysisThe results of the probabilistic sensitivity analysis, plotted on the cost-effectiveness plane (Figure 2) and the acceptability curve (Figure 3), show that edoxaban was cost-effective in 73% to 81% of the simulations run.
It was also cost-effective in the different subgroup analyses (see scenario results in Table 7).
Analysis of Scenarios
| Scenario | Cost per QALY (€) |
|---|---|
| INR testing site (80% hospital, 20% outpatient setting) | 12 700 |
| INR testing site (20% hospital, 80% outpatient setting) | 10 332 |
| Lower annual cost of INR monitoring for patients receiving VKA therapy | 17 892 |
| Subgroup of patients with CHADS2 stroke risk score ≥ 3 | 8127 |
| Subgroup of patients with TRT ≥ 60% | 16 170 |
| No loss of utility for patients treated with VKA | 17 026 |
TRT, time in therapeutic range; QALY, quality-adjusted life years; VKA, vitamin K antagonist.
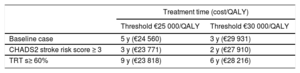
Finally, as indicated in Table 8, edoxaban becomes cost-effective after 3 to 5 years of treatment (2-3 years for patients with CHADS2 ≥ 3 and 6-9 years for those with a time in the therapeutic rage ≥ 60%) (Table 8).
Minimum Treatment Time Needed for Edoxaban to Be a Cost-Effective Alternative to Vitamin K Antagonists
| Treatment time (cost/QALY) | ||
|---|---|---|
| Threshold €25 000/QALY | Threshold €30 000/QALY | |
| Baseline case | 5 y (€24 560) | 3 y (€29 931) |
| CHADS2 stroke risk score ≥ 3 | 3 y (€23 771) | 2 y (€27 910) |
| TRT s≥ 60% | 9 y (€23 818) | 6 y (€28 216) |
QALY, quality-adjusted life years; TRT, time in therapeutic range.
Although VKAs are an effective treatment for preventing thromboembolic events in patients with NVAF, the need for frequent INR testing and dose adjustments carries a substantial risk of poor anticoagulation control and may also lead to underuse and poor adherence.17,51
This cost-effectiveness study, conducted in Spain, shows that compared with VKAs, edoxaban is a cost-effective option for preventing stroke in patients with NVAF and similar characteristics to the patients in the ENGAGE AF-TIMI 48 trial.19
This is the first cost-effectiveness study of edoxaban vs a VKA to be undertaken in Spain and the findings are similar to those reported for other countries.52–58
LimitationsThe results presented in this study are based on mathematical modeling, and to run the necessary simulations, we had to extrapolate short-term data (collected over a period of 2.8 years in the ENGAGE AF-TIMI 48 trial19) to the lifetime horizon of the current study and assume that the safety and efficacy of edoxaban remained constant over time. This is an inherent limitation that applies to any studies that need to calculate long-term treatment effects based on short-term clinical evidence mainly from phase III trials. Data from phase IV studies would confer greater external validity to these studies. Risk of stroke assessed using the CHADS2 scale, for example, was higher in the ENGAGE AF-TIMI 48 trial than in clinical practice59 (mean score of 2.8 vs 2.3).
The external validity of the current study may also be limited, as CHAD2S2-VASc scores are now used to assess AF stroke risk1 (and not CHADS2 scores like in the ENGAGE AF-TIMI 48 trial).
Other limitations are related to the variability of the data used and assumptions made about missing data. The need to model parameters from different sources is an inherent aspect of studies of chronic disease,60 and data on utilities, for example, often need to be drawn from international studies because of a shortage of national data. That said, in our case these data were applied to the 2 drugs and the relevant result in the analyses is the incremental ratio. We also accounted for potential variations and influences by conducting a probabilistic sensitivity analysis.
We were unable to investigate the potential influence of age as there was insufficient evidence for subgroup analyses. Nonetheless, efficacy and safety outcomes for edoxaban vs warfarin appear to be independent of age.61
One of the main assumptions in our model was the therapeutic equivalence of warfarin and acenocoumarol. Other cost-effectiveness studies performed in Spain have made similar assumptions about efficacy, safety, and resource utilization.47,62,63
Although different criteria were used to account for the loss of utility (or not) associated with VKA therapy in the cost-effectiveness analyses in the above studies,47,62,63 omission of this loss did not affect the results of the analyses.
The efficacy of edoxaban in our study may be underestimated, as in clinical practice, many patients with NVAF are given aspirin to prevent stroke or are not treated.64 Consideration of other types of costs associated with the long-term disability costs of stroke (eg, indirect health costs or productivity loss) would strengthen the cost-effectiveness of edoxaban.
The cost-effectiveness of edoxaban vs VKA therapy decreases with improved anticoagulation control,57 and therefore, in line with current recommendations, edoxaban can be considered to be particularly cost-effective in patients on VKA therapy with poor control (time in therapeutic range <60%).10
Considering the absence of randomized controlled trials directly comparing DOACs in patients with NVAF, differences observed indirectly through network meta-analyses should be confirmed in clinical practice.51 In brief, there are no reliable data for guiding the optimal use of one DOAC or another,65 although all DOACs appear to be a safe and effective alternative to VKAs.17 Phase IV studies will help to evaluate the sustainability of the widespread use of DOACs in the industrialized world.
CONCLUSIONSOver the past decade, DOACs have emerged as an alternative for preventing stroke and systemic embolism in patients with NVAF, remedying some of the limitations associated with VKAs. Edoxaban is as efficacious as VKAs in the treatment of NVAF and in addition it has a better safety profile. Despite its higher pharmacologic cost, it is a cost-effective option for the Spanish national health system.
FUNDINGStudy funded by Daiichi Sankyo.
CONFLICTS OF INTERESTJ.M. Rodríguez and P. Barja de Soroa work at Daiichi Sankyo. F. Pérez-Alcántara is a member of Oblikue, an independent consultancy firm that received funds to conduct the analysis. I. Lekuona, M. Anguita, and J.L. Zamorano received consultancy fees from Daiichi Sankyo.
- –
DOACs are an alternative to VKAs for the prevention of stroke in patients with NVAF.
- –
DOACs are at least as efficacious and safe as VKAs, but they are more convenient as they do not require regular INR testing and have fewer interactions with drugs and food.
- –
Despite their advantages, DOACs are used less than VKAs and the reason sometimes given for this is the difference in price.
- –
Edoxaban is the most recent DOAC authorized for use in NVAF in Spain.
- –
This is the first cost-effectiveness analysis of edoxaban vs a VKA for the treatment of NVAF in Spain.
- –
Edoxaban versus VKA therapy is a cost-effective option for NVAF.
.