Keywords
INTRODUCTION
Most left ventricular aneurysms (VA) occur as a result of myocardial infarction (MI)1 although some other possible causes are hypertrophic cardiomyopathy (HCM) with midventricular obstruction,2 heart injury/surgery, tuberculosis, Chagas' disease, anomalous origin of the left coronary artery in the pulmonary artery, rheumatic fever, sarcoidosis, and myocarditis.1,3
Congenital left ventricular aneurysm is a very rare disease entity usually diagnosed by exclusion once other possible etiologies have been ruled out.1 Its clinical presentation is very variable and there is no standard treatment due to its low prevalence. This means that controversy exists regarding indications for surgical treatment. The case of a woman with congenital left VA requiring surgical resection is reported.
CLINICAL CASE
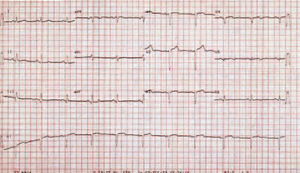
A 49-year-old woman without a family history of heart disease with a background of paroxysmal supraventricular tachycardias for 17 years. At 34 years old she was examined due to symptoms of diplopia with suspected transitory ischemic attack (TIA) without any other cardiovascular symptoms. Electrocardiogram (ECG) presented an image of old inferior and anteroseptal necrosis (Figure 1). Chest x-ray showed calcification on the cardiac apex whereas Tc-99m blood pool radionuclide ventriculography had already showed an aneurysmatic image on the lower wall.
Figure 1. 12-lead electrocardiogram presenting an image of old inferior and anteroseptal necrosis.
At 44 years old, without presenting cardiovascular symptomatology, she was assessed in the cardiology service for pathological ECG findings. Ergometry showed good functional capacity (8 MET), a 1-mm depression in the ST segment on the lower side with maximum effort and frequent ventricular extrasystoles (VE). Holter monitoring demonstrated scant supraventricular extrasystoles with repeat phenomena, very frequent VE in pairs and a series of nonsustained ventricular tachycardia. No alterations were detected with the initial transthoracic echocardiogram (TTE). Thallium myocardial perfusion tomography revealed a large perfusion defect in the anterior, apical, and lower sides, reversible during resting, except in the apical area. To clarify these conflicting findings, cardiac catheterization was performed demonstrating some normal coronary arteries and a calcified apical left ventricular aneurysmatic mass.
Following this, the patient was followed up on an outpatient basis, without presenting cardiovascular symptoms under medical treatment.
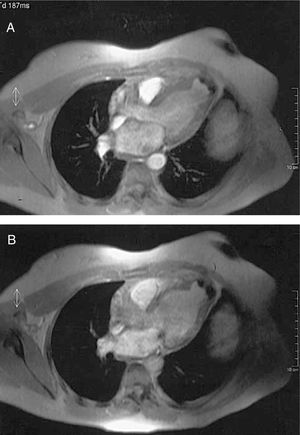
Given that the poor echocardiographic window hindered characterizing the aneurysm, cardiac magnetic resonance imaging (MRI) was done demonstrating an aneurysm in the LV apex measuring 257 mm2 with an 81-mm perimeter with calcification in the wall (Figure 2). In a later control TTE, a trend was noted toward dilatation of the left ventricular cavity (LVDD 62.3/LVSD 41.6 mm) with preserved systolic function (left ventricular ejection fraction 61%).
Figure 2. Cardiac turbo-gradient echo magnetic resonance imaging (TR=11 ms, TE=4 ms, a=20°) showing apical left ventricular aneurysm with calcified wall in systole (A) and diastole (B).
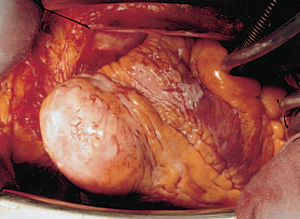
In the light of data indicative of ventricular remodelling, the patient underwent surgery. An aneurysmectomy and Dör endoaneurysmorrhaphy (Figure 3) were carried out, confirming the diagnosis of VA in the anatomopathological study. Patient evolution has been favorable at 24-month follow-up, while under treatment with beta-blockers, oral anticoagulation agents, and ACE inhibitors, with normalization of the ventricular diameters in postoperative TTE.
Figure 3. Aneurysmectomy sample.
DISCUSSION
Congenital VA is a rare clinical entity (0.4% of 750 cardiac necropsies)4 that is diagnosed once other more common etiologies have been ruled out. It is important to distinguish it from congenital left ventricular diverticulum (CLVD) with which it is frequently confused.5 The latter is characterized by presenting a narrow communication channel in the ventricular cavity, a wall made up of three differentiated layers and systolic contraction synchronized with the ventricle. In contrast, in aneurysms, the area joining the LV is wide, histologically they lack the myocardial muscle layer and present a single layer of fibroelastic tissue, at times calcified6; furthermore, during systole they show paradoxical expansion.5 In addition, whereas VA are usually isolated congenital defects, 70% of CLVD are associated with congenital midline thoracoabdominal defects and congenital cardiac malformations.7
The severity of the clinical manifestations of congenital VA varies widely from one patient to the other,3 and includes supraventricular and ventricular arrhythmias (as in our case),8,9 heart failure, peripheral embolism, endocarditis, cardiac rupture, tamponade,4 or even sudden death.3
Surgical resection is indicated in almost all patients to prevent these complications,7,8 although some groups defend a conservative attitude in asymptomatic patients and employ measures aimed at preventing endocarditis and embolism via oral antiplatelet or anticoagulation agents.3
In our case, despite involving a practically asymptomatic patient, the progressive dilatation of the ventricular cavities, together with the background of neurological symptoms of possible embolic origin, led to surgery being proposed as the treatment of choice to prevent future complications.
The presence of normal coronary arteries, the exclusion of previous AMI or HCM, as confirmed by histological study of the resected part, as well as other possible etiologies in her clinical history, allowed us to infer that the VA was most probably of congenital origin. This was supported by the onset of clinical manifestations at an early age and the presence of calcification in the aneurysmatic region which requires a certain amount of time to become established.
Several imaging techniques are useful in the diagnosis of VA, including chest x-ray, simple or contrast echocardiography, cardiac catheterization and MRI.3,4,7,10 In the present case, due to a poor echocardiographic window, the diagnosis of aneurysm escaped notice in the initial echocardiographic explorations. Magnetic resonance imaging was the technique that more precisely defined the cardiac morphology and function leading to the correct diagnosis of calcified apical VA via a non-invasive technique.
Received December 14, 2004.
Accepted for publication March 31, 2005.
Correspondence: Dr. R. Pérez-Fernández.
Servicio de Cardiología. Complexo Hospitalario de Pontevedra.
Mourente-Montecelo. 36071 Pontevedra. España.
E-mail: rula_perez@hotmail.com