To the Editor:
Positron emission tomography (PET) is currently the reference technique for determining whether viable myocardium is present because it is the only one that can use specific tracers for both flow (13NH3) and cardiac metabolism (18FDG). The following basic patterns for diagnosis of viable myocardium have been established1,2:
- Match pattern: low uptake of both tracers, suggestive of lack of viability in the necrotic region.
- Mismatch pattern: flow is reduced but metabolism is maintained, suggestive of viability in the necrotic area.
In this letter to the editor, we present the case of a 72-year-old patient with cardiovascular risk factors (dyslipidemia and a smoking habit), who was admitted to hospital for unstable angina. Anterior acute myocardial infarction (AMI) was diagnosed and treated with fibrinolytic agents. Catheterization revealed atheromatosis with multiple insignificant lesions and a lesion involving 90% of the middle left anterior descending (LAD) artery. According to the ventriculogram, severe anterolateral hypokinesia was present, with an ejection fraction (EF) of 67%.
The LAD artery was stented in a percutaneous transluminal coronary angioplasty procedure. The echocardiogram on discharge showed that the left ventricle was not dilated and that wall motion was normal (EF, 64%).
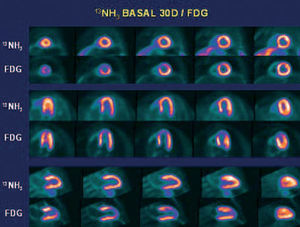
Thirty days post-AMI, myocardial viability was studied with 18FDG as a tracer and correlated with regional flow alterations measured with 13NH3 as a tracer. The findings of the study of flow with 13NH3 were almost normal, whereas decreased metabolism in the necrotic area was seen in the study with 18FDG (reverse mismatch pattern) (Figure 1).
Figure 1. Study of myocardial metabolism (18FDG), done 30 days after myocardial infarction, showing decreased glucose uptake in medial and apical segments of the anterior territories, although myocardial flow was almost normal (13NH3).
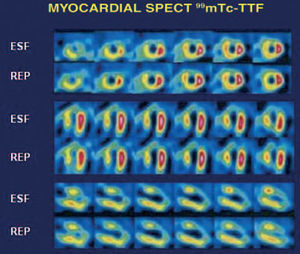
After 1 year, the patient had suffered no further acute coronary episodes. He underwent a single-photon emission computed tomography control study of the myocardium with 99mTc-tetrofosmin (8 mCi/22 mCi) and an exercise test with the cycle ergometer. The findings of exercise testing proved clinically and electrically negative, although the test was limited to 75% of maximum because of the effect of treatment with carvedilol (50 W; 4.3 MET). Anterior necrosis was documented in the myocardial perfusion study, with involvement of the apical and medial segments and no viable tissue or signs of proximal or distal ischemia (Figure 2).
Figure 2. Tomographic sections obtained from the postexercise study done with 99mTc-tetrofosmin, showing intense hypoperfusion in the medial and apical segments of the anterior territory, with no significant changes in the images taken at rest. These findings are compatible with anterior necrosis, with no viable tissue or signs of associated or distal ischemia.
Despite necrosis, the patient had normal coronary flow with low glucose uptake, a pattern that has been described as reverse mismatch. The pathophysiology of this condition seems to be multifactorial. Mechanisms such as dissolved fragments of thrombi causing microinfarction of distal vessels, use of other substrates during the acute phase of AMI, relative hyperemia, and the development of collateral vessels are all potentially implicated.3
The few studies that have been done on the reverse mismatch pattern have enrolled different types of patient. Nevertheless, the literature points to a prevalence of almost 30%.4 Myocardial histology was investigated in an animal model of myocardial infarction, and patchy necrosis was found in which a transmural area and mixture of myocardial fibrosis and viable tissue were observed, with structural alterations of the cardiomyocytes at the edges of the microinfarction.5
In conclusion, the reverse mismatch pattern, in which flow and cardiac metabolism (decreased metabolism) do not correlate, should be interpreted as "threatened" tissue that is probably unrecoverable when associated with patchy necrosis.