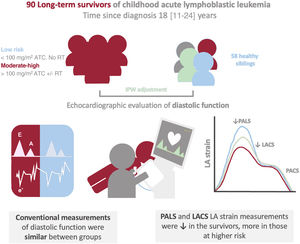
Survivors of childhood cancer might be at increased risk of diastolic dysfunction at follow-up due to exposure to cardiotoxic treatment. Although assessment of diastolic function is challenging in this relatively young population, left atrial strain might provide a novel insight in this evaluation. Our aim was to examine diastolic function in a cohort of long-term survivors of childhood acute lymphoblastic leukemia by using left atrial strain and conventional echocardiographic parameters.
MethodsLong-term survivors who were diagnosed at a single center between 1985 and 2015 and a control group of healthy siblings were recruited. Conventional diastolic function parameters and atrial strain were compared, and the latter was measured during the 3 atrial phases: reservoir (PALS), conduit (LACS) and contraction (PACS). Inverse probability of treatment weighting was used to account for differences between the groups.
ResultsWe analyzed 90 survivors (age, 24.6±9.7 years, time since diagnosis 18 [11-26] years) and 58 controls. PALS and LACS were significantly reduced compared with the control group: 46.4±11.2 vs 52.1±11.7; P=.003 and 32.5±8.8 vs 38.2±9.3; P=.003, respectively. Conventional diastolic parameters and PACS were similar between the groups. The reductions in PALS and LACS were associated with exposure to cardiotoxic treatment in age- and sex-adjusted analysis (≥ moderate risk, low risk, controls): 45.4±10.5, 49.5±12.9, 52.1±11.7; Padj=.003, and 31.7±9.0, 35.2±7.5, 38.2±9.3; Padj=.001, respectively.
ConclusionsLong-term childhood leukemia survivors showed a subtle impairment of diastolic function that was detected with atrial strain but not with conventional measurements. This impairment was more pronounced in those with higher exposure to cardiotoxic treatment.
Keywords
Acute lymphoblastic leukemia (ALL) is the most frequent type of cancer in the pediatric population. In the last few decades, antineoplastic therapies have increased survival rates, and current 5-year survival rates are above 90%.1 This improvement in prognosis has led to increased numbers of childhood leukemia survivors (CLSs) who are at considerable risk of developing asymptomatic cardiotoxicity and subsequent heart failure (HF) during long-term follow-up.2,3
Most studies including echocardiographic assessment of long-term CLSs have focused on the evaluation of left ventricular systolic function, with a high prevalence of asymptomatic and subclinical left ventricular systolic dysfunction (LVSD).4 Diastolic function may also be compromised due to the cardiotoxic effect of cancer therapies.5 However, its evaluation in long-term CLSs has yielded inconclusive results to date, which might be partially explained by more complex echocardiographic assessment and its association with age.6
Left atrial strain (LAS) has been proposed as a sensitive marker of diastolic dysfunction (DD) and has excellent correlation with invasive techniques for the assessment of left ventricular (LV) filling pressures.7,8 There are few data on the use of LAS in long-term CLSs. We hypothesized that LAS could be impaired in CLSs and, thus, might be useful to detect subtle changes in the diastolic function of these patients.
The purpose of this study was to assess diastolic function in a cohort of long-term CLSs by quantification of automated LAS and conventional parameters.
METHODSParticipantsThe current work was conducted within the framework of the CTOXALL study. Briefly, CTOXALL is a cross-sectional study of a single-center cohort of childhood ALL survivors diagnosed between 1985 and 2015 and a control group composed of healthy siblings. The aim of the CTOXALL study is to evaluate the prevalence of long-term cardiotoxicity in CLSs with novel echocardiographic parameters and biomarkers. We have previously reported a high prevalence of subclinical LVSD in these patients.4 The present study aimed to assess the added value of LAS to conventional echocardiographic parameters (figure 1). The CTOXALL study protocol was approved by the local clinical research ethics committee according to institutional and good clinical practice guidelines. Written informed consent was obtained from all participants, parents, or legal guardians.
Central illustration. Assessment of left atrial strain in a cohort of childhood ALL. A cohort of long-term childhood ALL showed impairment in diastolic function detected by left atrial strain but not by conventional parameters. This impairment was more marked in those at higher risk according to exposure to cardiotoxic treatment. ATC, anthracycline; IPW, inverse probability weighting; LA, left atrial; LACS, LA strain during the conduit phase; PACS, peak atrial contraction strain; PALS, peak atrial longitudinal strain; RT, radiotherapy.
Participants were evaluated from May 2019 to January 2022 at Hospital Universitario Reina Sofía (Cordoba, Spain). Survivors were eligible if they were diagnosed with ALL before the age of 18 years and had received the last anthracycline dose at least 3 years prior to their inclusion in the study. An individual with congenital heart disease (ventricular septal defect) was excluded. A sample of healthy siblings of survivors willing to participate was recruited as a comparison group.
Clinical assessmentAll patients underwent clinical evaluation. Exposure to cardiotoxic treatments and doses were collected from medical records. Cumulative anthracycline doses were converted to doxorubicin equivalents using previously described conversion factors: 0.6 for daunorubicin, 0.8 for epirubicin, and 10.5 for mitoxantrone.9 Radiotherapy exposure was considered when the heart region was involved, including total body irradiation. According to the European guidelines on cardio-oncology, patients exposed to a cumulative doxorubicin dose<5Gy radiotherapy dose and <100mg/m2 were classified as low risk. The remaining patients were classified as ≥ moderate risk.10
EchocardiographyAll studies were performed by qualified echocardiographers using the same equipment (EPIQ CVx and iE33, Philips Medical Systems, United States). Standard echocardiographic parameters were obtained in accordance with the latest guidelines.11,12 Manual tracing of the left atrium (LA) borders in the apical 4-chamber and 2-chamber views was used to measure LA volumes with the modified biplane Simpson method. LV diastolic function was evaluated following the American Society of Echocardiography/European Association of Cardiovascular Imaging (ASE/EACVI) algorithm, which is based on the following variables: mitral flow velocities, mitral annular e’ velocity, E/e’ ratio, peak velocity of tricuspid regurgitation jet, and LA maximum volume index (LAVI).13
For the present study, we performed a retrospective post hoc analysis of LAS using a semiautomated assessment with AutoStrain (TomTec-Arena, TomTec Imaging Systems, Germany). LAS was measured from a nonforeshortened apical 4-chamber view, as recommended in the ASE/EACVI consensus report on the standardization of deformation imaging.14
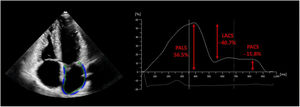
Ventricular end-diastole and R-wave in the electrocardiogram were used as the time reference to define the zero-baseline for LAS curves. LA function can be defined by 3 components: reservoir (from end-diastole to mitral valve opening), conduit from mitral valve opening, throughout diastasis to onset of atrial contraction) and contraction phase (from onset of atrial contraction to end-diastole). LAS was assessed during these 3 phases and was represented by peak atrial longitudinal strain (PALS), peak atrial contraction strain (PACS), and LA strain during the conduit phase (LACS), representing the reservoir, contractile, and conduit function of the LA, respectively (figure 2). After automatic calculation using the software, the operator manually adjusted the contours if needed. For simplicity, LAS measurements are reported as their absolute values.
Automated echocardiographic assessment of left atrial strain. Automatic tracing of the left atrium was performed from the apical 4-chamber view, as shown on the left of the image. The time reference to define the zero-baseline for left atrial strain (LAS) was set at ventricular end-diastole and R-wave in the electrocardiogram. The values of peak atrial longitudinal strain (PALS), left atrial strain during the conduit phase (LACS), and peak atrial contraction strain (PACS) are represented on the graphic on the right.
To test intraobserver and interobserver variability, 20 echocardiograms were randomly selected, and LAS was measured by the same investigator who performed the analysis and a second investigator, respectively.
Statistical analysisCategorical variables are presented as count (percentage) and continuous variables as mean±standard deviation or median [interquartile range] according to their distribution, which was assessed using the Shapiro-Wilk test and QQ plots. Survivors and controls were compared using the chi-square test or Fisher exact test for categorical data and the Student t-test or Mann-Whitney U test for continuous data, as appropriate.
General linear models were used to compare echocardiographic measurements between groups. Inverse probability of treatment weighting (IPW) was used to balance patient characteristics in the 2 groups.15 Propensity scores were computed using logistic regression with age, sex, body mass index, heart rate, and diastolic blood pressure as covariates. Standardized mean differences before and after the weighting were used to evaluate the balance. A difference of <10% was considered to indicate good balance. The distribution of the propensity score before and after the weighting was plotted to assess the degree of overlap between the 2 groups. Standard errors of the IPW linear regression coefficients were obtained using robust sandwich-type variance estimators.16
Univariable and multivariable linear regression models were used to compare echocardiographic parameters between ≥ moderate risk CLSs, low-risk CLSs and controls, with age and sex as covariables in the multivariable models.
Intraobserver and interobserver agreement were assessed using intraclass correlation coefficients and the Bland-Altman method, plotting the difference of 2 measurements (y-axis) against their mean (y-axis) for each subject. The limit of agreement was computed as the mean difference±1.96 standard deviation.
Statistical analyses were performed using the SPSS software (version 24; IBM Corp., United States) and R software (version 4.0.3; R Foundation for Statistical Computing, Austria).
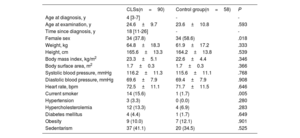
RESULTSParticipant characteristicsDuring the above-mentioned period, 170 patients with age <18 years were diagnosed with ALL at our center. A total of 52 patients had died before the start of the recruitment period. Among the 118 long-term survivors, 28 were not included (17 could not be reached, 10 refused to participate, and 1 had been diagnosed with congenital heart disease). Thus, the CLSs group was integrated by 90 patients.
The CLSs group had a median age at diagnosis of 4 [3-8] years and 34 (37.8%) were women. The mean age at recruitment was 24.6±9.7 years. The median time from diagnosis was 18 [11-26] years. The control group was composed of 58 healthy siblings. The characteristics of the unweighted groups are shown in table 1. Both groups were comparable in age, body measurements, and prevalence of risk factors, but there was a higher proportion of women in the control group (37.8% vs 56.6%; P=.018). The prevalence of sedentarism was high in both groups (41.1% vs 34.5%; P=.525). Smoking was more frequent in the CLSs group (15.6% vs 1.7%; P=.005). The variables used in the IPW models for the echocardiographic comparisons between the groups, including sex, were well balanced after the weighting, with standardized mean differences <10% for all the covariates (figure 1 of the supplementary data).
Characteristics of childhood acute lymphoblastic leukemia survivors and controls
| CLSs(n=90) | Control group(n=58) | P | |
|---|---|---|---|
| Age at diagnosis, y | 4 [3-7] | - | - |
| Age at examination, y | 24.6±9.7 | 23.6±10.8 | .593 |
| Time since diagnosis, y | 18 [11-26] | - | - |
| Female sex | 34 (37.8) | 34 (58.6) | .018 |
| Weight, kg | 64.8±18.3 | 61.9±17.2 | .333 |
| Height, cm | 165.6±13.3 | 164.2±13.8 | .539 |
| Body mass index, kg/m2 | 23.3±5.1 | 22.6±4.4 | .346 |
| Body surface area, m2 | 1.7±0.3 | 1.7±0.3 | .366 |
| Systolic blood pressure, mmHg | 116.2±11.3 | 115.6±11.1 | .768 |
| Diastolic blood pressure, mmHg | 69.6±7.9 | 69.4±7.9 | .908 |
| Heart rate, bpm | 72.5±11.1 | 71.7±11.5 | .646 |
| Current smoker | 14 (15.6) | 1 (1.7) | .005 |
| Hypertension | 3 (3.3) | 0 (0.0) | .280 |
| Hypercholesterolemia | 12 (13.3) | 4 (6.9) | .283 |
| Diabetes mellitus | 4 (4.4) | 1 (1.7) | .649 |
| Obesity | 9 (10.0) | 7 (12.1) | .901 |
| Sedentarism | 37 (41.1) | 20 (34.5) | .525 |
Continuous variables are presented as median (interquartile range) or mean±standard deviation. Categorical variables are presented as n (%). ALL: acute lymphoblastic leukemia; CLSs: childhood acute lymphoblastic leukemia survivors.
The data are presented as No. (%), mean±standard deviation, or median [interquartile range].
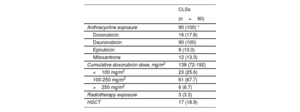
The details of the cardiotoxic treatments received by the CLSs are shown in table 2. All of them were exposed to anthracyclines. The mean isotoxic cumulative anthracycline dose was 138 [72-192mg/m2]. Three (3.3%) patients received thoracic radiation and 17 (18.9%) underwent hematopoietic stem cell transplantation. In all, 23 (25.6%) survivors were classified as low risk according to current recommendations. The prevalence of asymptomatic LVSD (left ventricle ejection fraction [LVEF] <50%) was 12.2% and of subclinical LVSD (global longitudinal strain [GLS] <18.5%) was 26.6%.
Cardiotoxic therapies received by the survivors
| CLSs | |
|---|---|
| (n=90) | |
| Anthracycline exposure | 90 (100) * |
| Doxorubicin | 16 (17.8) |
| Daunorubicin | 90 (100) |
| Epirubicin | 9 (10.0) |
| Mitoxantrone | 12 (13.3) |
| Cumulative doxorubicin dose, mg/m2 | 138 (72-192) |
| <100 mg/m2 | 23 (25.6) |
| 100-250 mg/m2 | 61 (67.7) |
| >250 mg/m2 | 6 (6.7) |
| Radiotherapy exposure | 3 (3.3) |
| HSCT | 17 (18.9) |
ALL, acute lymphoblastic leukemia; CLSs, childhood acute lymphoblastic leukemia survivors; HSCT, hematopoietic stem cell transplantation.
Data are presented as No. (%).
Conventional echocardiographic diastolic measurements were available and feasible in all patients. There were no cases of DD or undetermined diastolic function according to the ASE/EACVI algorithm.
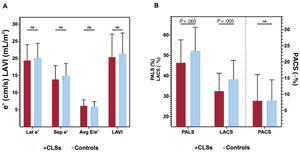
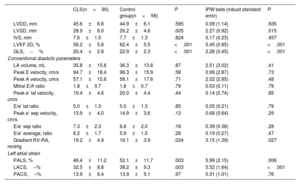
Comparison of echocardiographic parameters between survivors and controlsConventional diastolic measurementsConventional diastolic function parameters based on mitral annulus tissue doppler and LAVI values were within the normal limits and there were no differences between groups: peak e’ lateral velocity (cm/s) (19.4±4.6 vs 20.0±4.4; P=.439), e’ septal velocity in cm/s (13.9±4.0 vs 14.9±3.6; P=.131), E/e’ average ratio (6.2±1.7 vs 5.9±1.5; P=.279), and LAVI (ml/m2) (20.65±6.8 vs 21±6.1; P=.277). After IPW adjustment, these diastolic parameters remained similar between the groups. The gradient between right ventricle and right atrium was within the normal limits in both groups but was slightly higher in survivors (19.2±4.9 vs 16.1±3.9; P=.024). This difference remained significant after IPW adjustment (table 3, figure 3A).
Echocardiographic measurements of CLSs and controls
| CLS(n=90) | Control group(n=58) | P | IPW beta (robust standard error) | P | |
|---|---|---|---|---|---|
| LVDD, mm | 45.6±6.8 | 44.9±6.1 | .595 | 0.09 (1.14) | .935 |
| LVSD, mm | 28.9±6.0 | 26.2±4.6 | .005 | 2.27 (0.92) | .015 |
| IVS, mm | 7.6±1.5 | 7.7±1.3 | .824 | 0.17 (0.23) | .457 |
| LVEF 2D, % | 56.2±5.8 | 62.4±5.5 | <.001 | 5.45 (0.95) | <.001 |
| GLS,−% | 20.4±2.8 | 22.9±2.3 | <.001 | 2.28 (0.45) | <.001 |
| Conventional diastolic parameters | |||||
| LA volume, mL | 35.8±15.6 | 36.3±13.8 | .87 | 2.51 (3.02) | .41 |
| Peak E velocity, cm/s | 94.7±18.4 | 96.3±15.9 | .58 | 0.99 (2.87) | .73 |
| Peak A velocity, cm/s | 57.1±15.6 | 58.1±17.6 | .71 | 2.02 (2.85) | .48 |
| Mitral E/A ratio | 1.8±0.7 | 1.8±0.7 | .79 | 0.03 (0.11) | .79 |
| Peak e’ lat velocity, cm/s | 19.4±4.6 | 20.0±4.4 | .44 | 0.14 (0.74) | .85 |
| E/e’ lat ratio | 5.0±1.3 | 5.0±1.3 | .85 | 0.05 (0.21) | .79 |
| Peak e’ sep velocity, cm/s | 13.9±4.0 | 14.9±3.6 | .13 | 0.68 (0.64) | .29 |
| E/e’ sep ratio | 7.3±2.3 | 6.8±2.0 | .18 | 0.39 (0.36) | .28 |
| E/e’ average, ratio | 6.2±1.7 | 5.9±1.5 | .28 | 0.19 (0.27) | .47 |
| Gradient RV-RA, mmHg | 19.2±4.9 | 16.1±3.9 | .024 | 3.15 (1.39) | .027 |
| Left atrial strain | |||||
| PALS, % | 46.4±11.2 | 52.1±11.7 | .003 | 5.99 (2.15) | .006 |
| LACS,–% | 32.5±8.8 | 38.2±9.3 | .003 | 5.52 (1.64) | <.001 |
| PACS,–% | 13.9±6.4 | 13.9±5.1 | .97 | 0.31 (1.01) | .76 |
CLS, childhood acute lymphoblastic leukemia survivors; GLS, global longitudinal strain; IPW, inverse probability weighting; LVDD, left ventricular diastolic diameter; LVSD, left ventricular systolic diameter; IVS, interventricular septum; LA, left atrium; LACS, left atrial strain during the conduit phase; LVEF, left ventricular ejection fraction; RV-RA gradient, pressure gradient between right ventricle and right atrium; PALS, peak atrial longitudinal strain; PACS, peak atrial contraction strain.
Data are presented as mean±standard deviation.
Comparison of echocardiographic parameters between survivors and controls. Traditional echocardiographic parameters of diastolic function showed no difference between survivors and controls (A). Peak atrial longitudinal strain (PALS) and left atrial strain during conduit phase (LACS) were significantly lower in the survivor group. Peak atrial contraction strain (PACS) was similar between groups (B). Avg: average; CLS, childhood leukemia survivors; Lat: lateral; LAVI, left atrial volume index; ns, non significant; sep, septal.
Automated LAS parameters (PALS, PACS and LACS) were available in all patients and were feasible in 91.9% of them. PALS and LACS were within the normal limits but were significantly lower in survivors than in controls (46.4±11.2 vs 52.1±11.7, P=.003, and 32.5±8.8 vs 38.2±9.3; P=.003, respectively). PACS was also within the normal range but was similar in the 2 groups (13.9±6.4 vs 13.9±5.1; P=.971). Similarly, after IPW adjustment, PALS and LACS, but not PACS, were reduced compared with the control group (table 3, figure 3B).
There was a weak correlation between PALS and GLS (r=0.29; P=.001), a moderate correlation between LACS and GLS (r=0.35; P=.001) and no correlation between PACS and GLS (r=0.02; P=.801). After multivariable adjustment by age, sex and impaired GLS, PALS and LACS, differences between survivors and controls remained significant (PALS P=.030; LACS P=.007). As in the unadjusted and IPW-adjusted analysis, there was no significant difference in PACS between the groups (P=.894).
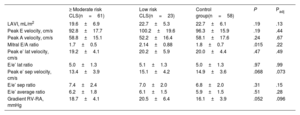
Comparison of echocardiographic parameters according to cardiotoxicity riskAmong the CLSs, 23 (25.6%), 61 (67.7%) and 6 (6.7%) patients could be classified as low-, moderate-, and high-risk, respectively, according to the European guidelines on cardio-oncology.10 Considering this classification, we formed 3 groups for the subsequent analysis: ≥ moderate-risk CLSs (n=67), low-risk CLSs (n=23), and controls (n=58). In the unadjusted analysis, most conventional echocardiographic diastolic measurements were similar among the 3 groups, except for E/A ratio and peak systolic right ventricle-right atrial gradient (RV-RA), which were higher in the low-risk CLSs group. After adjustment by age and sex, all the conventional diastolic parameters were comparable between the 3 groups (table 4).
Conventional echocardiographic measurements according to cardiotoxicity risk
| ≥ Moderate risk CLS(n=61) | Low risk CLS(n=23) | Control group(n=58) | P | Padj | |
|---|---|---|---|---|---|
| LAVI, mL/m2 | 19.6±6.9 | 22.7±5.3 | 22.7±6.1 | .19 | .13 |
| Peak E velocity, cm/s | 92.8±17.7 | 100.2±19.6 | 96.3±15.9 | .19 | .44 |
| Peak A velocity, cm/s | 58.8±15.1 | 52.2±16.4 | 58.1±17.6 | .24 | .67 |
| Mitral E/A ratio | 1.7±0.5 | 2.14±0.88 | 1.8±0.7 | .015 | .22 |
| Peak e’ lat velocity, cm/s | 19.2±4.1 | 20.2±5.9 | 20.0±4.4 | .47 | .49 |
| E/e’ lat ratio | 5.0±1.3 | 5.1±1.3 | 5.0±1.3 | .97 | .99 |
| Peak e’ sep velocity, cm/s | 13.4±3.9 | 15.1±4.2 | 14.9±3.6 | .068 | .073 |
| E/e’ sep ratio | 7.4±2.4 | 7.0±2.0 | 6.8±2.0 | .31 | .15 |
| E/e’ average ratio | 6.2±1.8 | 6.1±1.5 | 5.9±1.5 | .51 | .28 |
| Gradient RV-RA, mmHg | 18.7±4.1 | 20.5±6.4 | 16.1±3.9 | .052 | .096 |
CLS, childhood acute lymphoblastic leukemia survivors; IPW, inverse probability weighting; LAVI, left atrial volume index; RV-RA gradient, pressure gradient between right ventricle and right atrium.
Data are presented as mean±standard deviation.
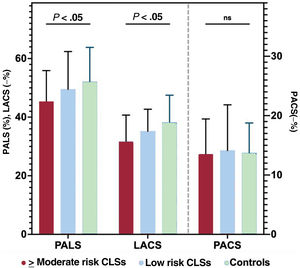
Conversely, statically significant differences were found in LAS measurements, since there was a progressive decline in PALS and LACS values according to cardiotoxic treatment exposure: PALS (%): 45.4±10.5, 49.5±12.9, 52.1±11.7; P=.007; and LACS (−%): 31.7±9.0, 35.2±7.5, 38.2±9.3; P=.001, for ≥ moderate-risk CLSs, low-risk CLSs and controls, respectively. These differences remained significant after adjustment by age and sex (PALS Padj=.003; LACS Padj=.001). PACS was similar between the 3 groups in the unadjusted and adjusted analysis (figure 4).
Comparison of left atrial strain parameters according to cardiotoxicity risk. Statistically significant differences were found in left atrial strain measurements between the risk groups: peak atrial longitudinal strain (PALS) and left atrial strain during conduit phase (LACS) progressively decelined according to exposure to cardiotoxic treatment. Peak atrial contraction strain (PACS) was similar between the 3 groups. CLS, childhood leukemia survivors; ns, non significant.
The intraclass correlation coefficients for intraobserver agreement were 0.97 (95% confidence interval [95%CI], 0.93-0.99), 0.95 (95%CI, 0.89-0.98), and 0.98 (95%CI, 0.97-0.99) for PALS, LACS, and PACS, respectively. The intraclass correlation coefficients for interobserver agreement were 0.93 (95%CI, 0.84-0.97), 0.90 (95%CI, 0.76-0.97), and 0.97 (95%CI, 0.96-0.98). Bland-Altman plots are shown in figure 2 of the supplementary data.
DISCUSSIONIn this study, we evaluated diastolic function in a cohort of long-term survivors of childhood ALL using conventional echocardiographic measurements and LAS. The main findings were the following: a) CLSs had reduced LAS values compared with their healthy siblings; b) this reduction was more pronounced in survivors with higher risk according to treatment exposure; c) conversely, conventional echocardiographic diastolic parameters were similar between the groups and among cardiotoxicity risk categories.
Until recently, anthracycline-induced cardiotoxicity was almost synonymous to LVSD, as defined by a decline in LVEF.17–19 In the last few years, major advances in diagnostic tools, in the context of a deeper knowledge of the molecular mechanisms involved in cardiotoxicity, have allowed us to move beyond a “LVEF-centric” view in favor of a more integrated approach.20,21 In this respect, biomarkers and LV GLS measurement permit the early detection of subclinical cardiotoxicity in patients with normal LVEF and, thus, are recommended in the periodic monitoring of patients undergoing cardiotoxic treatments.10,22–24 Additionally, worsening of LV diastolic function that persists over time has been observed in breast cancer patients treated with doxorubicin.25
Diastolic dysfunction based on conventional echocardiographic parametersThe risk of LVSD and HF in long-term survivors of childhood cancer is clearly established, but there is a lack of agreement regarding LV diastolic function impairment in these patients.26–28 Indeed, there is marked variation among the studies assessing the prevalence of DD in childhood cancer survivors, ranging from 0% to 29%.27,29,30 This variation seems to be fundamentally determined by 3 factors: the age of the survivors at the time of the echocardiographic evaluation, the percentage of patients exposed to mediastinal radiotherapy, and the parameters used to define DD. Christiansen et al.27 found a prevalence of DD of 29% in a cohort of 125 survivors of childhood lymphoma (median age at exam 33 years). Diastolic dysfunction definition was based on a single parameter (reduction in septal and/or lateral e’) and was particularly frequent in patients exposed to mediastinal radiotherapy, which were as high as 50%. In contrast, Slieker et al.31 failed to find diastolic function impairment in 546 pediatric survivors of childhood cancer. Survivors were younger (median age at assessment 14 years) and only 12% of them received mediastinal radiotherapy. In line with the latter study, we found no cases of DD based on conventional echocardiographic parameters, which is consistent with the relatively young age of the participants (mean age 25 years) with exceptional exposure to mediastinal radiotherapy (only 3 patients). Through a mechanism of microvascular damage and generation of reactive oxygen species, radiation might lead to interstitial fibrosis causing a compliance reduction in the LV walls. This leads to a 7-fold higher risk of developing of DD in patients treated with radiotherapy compared with the general population.32 Through the production of reactive species and free radicals and the inhibition of topoisomerase IIβ, anthracyclines lead to myocyte cell death and interstitial fibrosis. These disruptions generate ventricular wall stiffness, impaired relaxation and, eventually, elevated filling pressure and DD.33,34 Indeed, according to the theory of senescent cell accumulation, childhood cancer survivors are believed to have an accelerated aging-like phenotype.35 The varying prevalence of DD reported in the literature could therefore be interpreted as pictures of different stages of a dynamic phenomenon that is mainly driven by cellular senescence and that seems to be enhanced by different exposures, such as mediastinal radiotherapy and cumulative anthracycline dose. Due to the limitations of the multiparametric assessment of diastolic function36 and the overlap between normal and pathologic values, conventional echocardiographic parameters might be insufficient to detect the earliest stages of DD in childhood cancer survivors.
Diastolic dysfunction based on left atrial strainLAS has been proposed as a single, sensitive and reproducible measure of DD.37,38 Although this measurement is influenced by LV GLS, it has been proven to be independently associated with LV filling pressures.39,40 Accordingly, we found that there was a mild to moderate correlation between PALS/LACS and GLS, and that these measurements remained significantly reduced in the survivors, regardless of the presence of abnormal GLS. Additionally, LA wall fibrosis has been associated with reduced PALS41 and is a predictor of mortality and hospitalizations in patients with HF.42 PACS corresponds to active atrial contraction and seems to be less associated with filling pressures and diastolic function.43 We speculate that the thinner left atrial wall thickness and the differential atrial cardiomyocyte phenotype might be associated with a lower susceptibility of atrial myocardium to anthracycline-induced injury.44
There are limited data on LAS in long-term survivors of childhood cancer. Loar et al.45 evaluated LAS in a cohort of 45 pediatric survivors of childhood cancer (median age 11.8 years) and found a reduction in PALS and LACS compared with a group of healthy controls. No differences in conventional diastolic measurements or PACS were found between the groups. Patients exposed to ≥ 250mg/m2 anthracycline doses had the lowest values of PALS. Despite the younger age of the participants in the study, these findings are in line with those observed in our study and altogether support the hypothesis that there are subtle alterations in the diastolic function of childhood cancer survivors that can be detected by LAS in adolescence and early adulthood. We speculate that this subtle diastolic impairment, which seems to be more pronounced in patients exposed to higher doses of anthracyclines, might constitute an early, subclinical stage of DD that might worsen with aging-related changes in LV filling, ultimately leading to an increased risk of DD and overt HF over time.46,47
Strengths and limitationsTo our knowledge, this is the largest study to evaluate DD in long-term survivors of childhood cancer with a comprehensive approach applying both the ASE/EACVI multiparametric algorithm and LAS measurements. Although the cross-sectional design of the study prevented us from evaluating the prognostic value of LAS impairment in these patients, it allowed us to depict novel findings in the context of continuous advancement in cardiac imaging. Of note, that despite a mean follow-up of almost 20 years since diagnosis, most participants were young adults at the time of the evaluation, which may limit the accuracy of conventional diastolic parameters in our sample. Conversely, a strength of the study is the use of more robust strain measurements. Furthermore, although echocardiograms were not analyzed by a core lab, automated software was used for quantification. Another strength of the study is the inclusion of a homogeneous population of ALL survivors with similar protocol-based treatments, reducing confusion bias secondary to heterogeneity in age of presentation, treatment regimens, and other unmeasurable disease-related factors. However, this came at the cost of a low percentage of patients exposed to radiotherapy, a factor that is strongly associated with DD. In this respect, the composition of the control group of healthy siblings reduced unmeasurable genetic and environmental factors, but resulted in sex differences between the 2 groups, which could have led to bias. Irrespective of this consideration, inverse probability weighting resulted in a good balance of sex and the other relevant covariates.
CONCLUSIONSLong-term survivors of childhood ALL showed impaired diastolic function compared with healthy siblings when evaluated with LAS, but not when evaluated by conventional parameters. This impairment was more pronounced in survivors exposed to more aggressive treatments. Longitudinal studies are needed to determine whether this finding constitutes an early stage of DD in these patients.
- -
Long-term survivors of ALL treated with anthracyclines are at risk of developing diastolic dysfunction.
- -
Conventional echocardiographic parameters of diastolic function might not be sensitive enough to detect early stages of DD.
- -
LAS seems to be impaired in cancer survivors at pediatric age.
- -
Diastolic dysfunction is an age-related process that might be accelerated in CLS due exposure to cardiotoxic treatments.
- -
LAS is reduced in long-term survivors of childhood cancer compared with controls. Therefore, LAS might be an early marker of DD in these patients.
- -
Prospective and translational studies are needed to corroborate the predictive value of LAS and its association with molecular hallmarks of aging.
The CTOXALL study received research grants from the Sociedad Española de Cardiología (Madrid, Spain) and the Sociedad Andaluza de Cardiología (Granada, Spain).
AUTHORS’ CONTRIBUTIONSC. Fernández-Aviles and R. González-Manzanares contributed equally to the present work as first authors. J.C. Castillo and M. Pan contributed equally as senior authors. Conceptualization: C. Fernández-Aviles, R. González-Manzanares, J.C. Castillo, D. Mesa, J.R. Molina. Methodology: R. González-Manzanares, S. Ojeda, M. Pan. Formal analysis: R. González-Manzanares. Investigation: R. González-Manzanares, J.R. Molina, C. Fernández-Aviles, G. Heredia, A. Resúa, F.J. Hidalgo, J. López-Aguilera. Resources: D. Mesa, M. Anguita, M. Pan. Data curation: R. González-Manzanares, C. Fernández-Aviles, G. Heredia. Writing—original draft: C. Fernández-Aviles, R. González-Manzanares. Writing—review and editing: J.C. Castillo, S. Ojeda, F. Hidalgo, J. López-Aguilera, D. Mesa, M. Anguita, M. Pan. Supervision: J.R. Molina, D. Mesa, M. Anguita, J.C. Castillo, S. Ojeda, M. Pan. Project administration: J.C. Castillo. Funding acquisition: R. González-Manzanares.
CONFLICTS OF INTERESTThe authors have no conflicts of interest to declare.