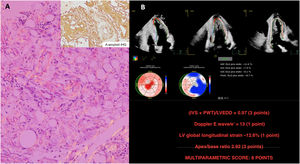
A 43-year-old man with chronic psoriatic arthritis was admitted for obstructive uropathy caused by an intraabdominal mass, which showed the characteristic Congo red stain and apple green birefringence (figure 1A). The patient's long-lasting inflammatory arthritis, high serum A protein and immunohistochemistry (IHQ) were compatible with a diagnosis of secondary A-amyloid (AA) systemic amyloidosis.
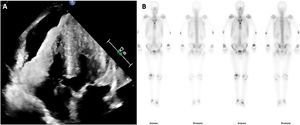
An echocardiogram revealed a severely thickened myocardium (figure 1B, video 1 of the supplementary data), resulting in a significant outflow tract gradient (video 2A of the supplementary data) and moderate pericardial effusion (figure 2A). In line with the latest amyloidosis position statement, multiparametric echo-score confirmed the diagnosis (figure 1B; DLG: global longitudinal strain; IVS: interventricular septum; LVEDD: left ventricle end-diastolic diameter; PWT: posterior wall thickness), while the AA nature of the cardiac involvement was supported by the normal kappa and lambda immunochain values, ratio, and a 0 Perugini score in bone scintigraphy (figure 2B).
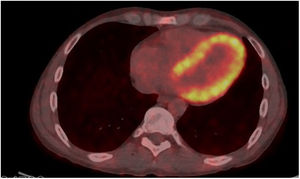
A [18F]-fluorodeoxyglucose-positron emission tomography/computed tomography ([18F]-FDG-PET/CT) had been performed a year before the admission, focused on joint inflammation evaluation with optimal dietary preparation. Subsequent analysis of this scan revealed noticeable [18F]-FDG uptake in the left ventricle myocardium (figure 3).
Specific intravascular volume-directed therapy eliminated the gradient (video 2B of the supplementary data) and tofacitinib-tocilizumab was initiated, with no cardiovascular symptoms reported at 6 months of follow-up.
Of note, the [18F]-FDG uptake might have brought forward both cardiac involvement and the diagnosis of systemic amyloidosis in this patient, whose informed consent was obtained.
This is the first documented case of [18F]-FDG-PET/CT cardiac uptake in AA amyloidosis, although it was previously reported for light chain and transthyretin-mediated types and in PET/CTs with amyloid-directed tracers such as [18F]-florbetaben.
FUNDINGNo funding.
ETHICAL CONSIDERATIONSInformed consent was obtained.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONSAll authors participated in the writing of the manuscript and supervised its final content.
CONFLICTS OF INTERESTNone.