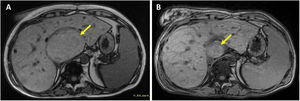
The present report concerns a 32-year-old woman with complex univentricular congenital heart disease (pulmonary and tricuspid atresia) treated with an extracardiac Fontan procedure who was being assessed for heart transplant (HTx) suitability; all relevant consent forms were signed. Vena cava and pulmonary conduit and artery pressures were 15mmHg while the transhepatic gradient was 4mmHg. Angiography revealed multiple venovenous and aortopulmonary collaterals that were not amenable to percutaneous occlusion due to their small size. The patient had moderate Fontan-associated liver disease (Child-Pugh B8) and posthepatic portal hypertension (small esophageal varices, splenomegaly, and thrombocytopenia). Liver magnetic resonance showed multiple hepatocellular adenomas; the largest was 60 × 80 × 78mm (figure 1A, arrow). Biopsy demonstrated METAVIR stage F3-4 fibrosis in the liver parenchyma and no malignancy of the main adenoma. After evaluation by the hepatology department, the patient was considered suitable for isolated HTx.
The transplant was conducted via the bicaval technique but was complicated by significant surgical bleeding that required delayed sternal closure after 48hours. Subsequently, the patient was stable with a well-functioning graft.
Liver resonance 1 year after the transplant showed a significant reduction in adenomas; the largest measured 35mm in size (figure 1B). Three years after the transplant, the main adenoma measured 30mm and there was no significant portal hypertension while liver function was similar (Child-Pugh A6). The adenoma regression was probably at least partly due to the restoration of the biventricular circulation and normalization of the posthepatic pressure. Although the liver biopsy was not repeated, it seems that the Fontan-associated liver disease probably stabilized after the HTx and even showed signs of reversing.
FUNDINGNo funding received.
ETHICAL CONSIDERATIONSInternational recommendations on clinical research (Declaration of Helsinki) have been respected. Sex and gender variables have been considered, in accordance with Sex and Gender Equity in Research (SAGER) guidelines. The patient provided signed informed consent.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this work.
AUTHORS’ CONTRIBUTIONSAll authors meet the requirements for authorship and contributed equally.
CONFLICTS OF INTERESTNone.
The authors would like to thank Ana Elvira González García, José Ruiz Cantador, Pablo Merás Colunga, Santiago Jiménez Valero, Óscar González Fernández, and Adriana Rodríguez Chaverri for their contribution to this manuscript.