There are scarce data on the factors associated with impaired functional status after transcatheter aortic valve replacement (TAVR) and its clinical impact. This study aimed to determine the incidence, predictors, and prognostic implications of impaired functional class (NYHA class III-IV) following TAVR.
MethodsThis multicenter study included 3462 transarterial TAVR patients receiving newer generation devices. The patients were compared according to their NYHA class at 1 month of follow-up (NYHA I-II vs NYHA III-IV). A multivariate logistic regression was performed to identify the predictors of 30-day NYHA class III-IV. Patient survival was compared with the Kaplan-Meier method and factors associated with decreased survival were identified with Cox regression analysis.
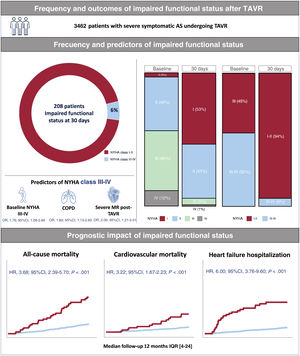
ResultsThe mean age of the study population was 80.3±7.3 years, with 47% of women, and a median Society of Thoracic Surgeons score of 3.8% [IQR, 2.5-5.8]. A total of 208 patients (6%) were in NYHA class III-IV 1 month after TAVR. Predictors of 30-day NYHA class III-IV were baseline NYHA class III-IV (OR, 1.76; 95%CI, 1.08-2.89; P=.02), chronic pulmonary obstructive disease (OR, 1.80; 95%CI, 1.13-2.83; P=.01), and post-TAVR severe mitral regurgitation (OR, 2.00; 95%CI, 1.21-3.31; P<.01). Patients in NYHA class III-IV 1 month after TAVR were at higher risk of death (HR, 3.68; 95%CI, 2.39-5.70; P<.01) and heart failure-related hospitalization (HR, 6.00; 95%CI, 3.76-9.60; P<.01) at 1-year follow-up.
ConclusionsUp to 6% of contemporary TAVR patients exhibited an impaired functional status following TAVR. Worse baseline NYHA class, chronic pulmonary obstructive disease, and severe mitral regurgitation predicted 30-day NYHA class III/IV, and this determined a higher risk of mortality and heart failure hospitalization at 1-year follow-up. Further studies on the prevention and treatment optimization of patients with impaired functional status after TAVR are needed.
Keywords
Transcatheter aortic valve replacement (TAVR) has become a well-established alternative for treating severe symptomatic aortic stenosis (AS), regardless of surgical risk.1,2 Several randomized clinical trials have consistently shown enhanced survival, quality of life, and functional class following TAVR.3,4
The third Valve Academic Research Consortium consensus definition for general outcome from the patient perspective requires a New York Heart Association (NYHA) class ≤ II at 1 year of follow-up for outcome to be described as favorable. Conversely, NYHA class III and IV define an acceptable and unfavorable outcome, respectively.5 Futility, defined as a lack of medical efficacy (improvement of symptoms) or meaningfully improved survival (1 year), is a matter of concern in the field of transcatheter valve interventions,2 especially among the highest-risk patients whose prognosis is influenced by older age and high comorbidity burden. Therefore, an appropriate preprocedural risk assessment is recommended for all patients undergoing TAVR.1,2
Lack of clinical improvement at 1 year of follow-up is present in more than 10% of patients undergoing TAVR and is associated with a poorer prognosis.6–8 However, earlier detection of suboptimal functional improvement following TAVR would be important to identify the potential causes and implement measures for improving clinical outcomes. The objectives of our study were to evaluate the incidence, predictors, and clinical impact of impaired functional class early (30 days) after TAVR.
METHODSStudy populationThis was a multinational, multicenter, observational nonprespecified retrospective analysis of prospectively collected data. A total of 4643 patients from 11 centers from Canada, France, Italy, and Spain who underwent TAVR between 2014 and 2022 were screened for inclusion in the study. Patients who died within the first 30 days after TAVR, those whose length of stay after the procedure was≥30 days, and those undergoing a transapical or transaortic approach were excluded from the analysis (435 patients, 9%). In 746 (16%) patients, NYHA class at 1 month of follow-up had not been recorded and they were therefore excluded from the analysis. Discharged patients rehospitalized for heart failure (HF) within the first month after TAVR were classified as NYHA class IV.
Each local heart team assessed the indications for TAVR, device type, and procedural approach based on an extensive clinical and anatomical preoperative assessment. The transfemoral approach was used by default, and alternative transarterial (transcarotid, transsubclavian/axillary) access was reserved for patients with unfavorable iliofemoral anatomy. This study was conducted according to the ethics committee of each participating center, and all patients provided signed informed consent for the procedures.
Data collectionBaseline, procedural, and follow-up data were prospectively collected in each dedicated local TAVR database. The patients’ pre-TAVR work-up and subsequent follow-up were performed according to local protocols. Information on NYHA class at 30 days was required for inclusion in this study. The patients’ vital status was updated after each medical contact with recording of the date of the last contact for each patient.
Statistical analysisContinuous variables are expressed as mean±standard deviation or median [Q1-Q3] according to the normality of data distribution assessed with the Shapiro-Wilk test. Categorical variables are expressed as number (%). The chi-square test or Fisher exact test were used to compare categorical variables. The Student t test or the Mann-Whitney U test were used to compare continuous variables. Factors associated with 1-month NYHA class III-IV were identified using logistic regression analysis. The variables to be analyzed were chosen based on previous causal knowledge and their association was assessed with univariable logistic regression analysis. Variables showing an association (P<.10) in the univariable analysis were included in the multivariable model.
For 1-year survival analysis, the Kaplan-Meier method was used to obtain event curves. The difference between the probability of event occurrence was assessed with the log-rank test. A Cox proportional hazards regression analysis was performed to evaluate the impact of 1-month NYHA class III-IV on mortality and HF hospitalization at 1-year follow-up. Models were adjusted for baseline confounders based on prior causal knowledge. A 2-sided P<.05 was considered significant for all statistical tests. All data were analyzed using the statistical package STATA version 15.0 (StataCorp LP, College Station, United States).
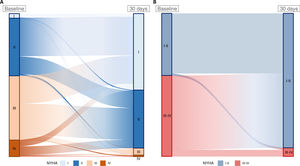
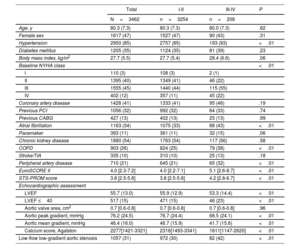
RESULTSA total of 3462 patients (80.3±7.3 years; 47% female) were included in the analysis. The median STS and EuroSCORE II were 3.8 [2.5-5.8]% and 4 [2.3-7.2]%, respectively. Among them, 2765 patients (80%), showed an improvement of at least 1 degree in their NYHA functional class; 611 patients (18%) reported no change; and 86 (2%) showed a deterioration. Regarding the classification in class I-II or III-IV, 1798 patients (52%) showed an improvement from NYHA III-IV to NYHA I-II; 1616 (47%) remained in the same group (90% I-II and 10% III-IV); and 48 (1%) showed a deterioration of their clinical status from NYHA I-II to NYHA III-IV (figure 1, table 1 of the supplementary data). Table 1 summarizes the baseline clinical and echocardiographic characteristics of the study population according to the NYHA class (I-II vs III-IV) at 1 month of follow-up.
Changes in New York Heart Association (NYHA) class from baseline to 1 month of follow-up. A: change from stage (NYHA I-IV). Improvement: 2765 patients (80%); no change 611 patients (18%); worsening: 86 patients (2%). B: change from group (NYHA I-II vs III-IV). Improvement: 1798 patients (52%); no change: 1616 patients (47%); worsening: 48 (1%) patients.
Baseline characteristics
| Total | I-II | III-IV | P | |
|---|---|---|---|---|
| N=3462 | n=3254 | n=208 | ||
| Age, y | 80.3 (7.3) | 80.3 (7.3) | 80.0 (7.3) | .62 |
| Female sex | 1617 (47) | 1527 (47) | 90 (43) | .31 |
| Hypertension | 2950 (85) | 2757 (85) | 193 (93) | <.01 |
| Diabetes mellitus | 1205 (35) | 1124 (35) | 81 (39) | .23 |
| Body mass index, kg/m2 | 27.7 (5.5) | 27.7 (5.4) | 28.4 (6.6) | .06 |
| Baseline NYHA class | <.01 | |||
| I | 110 (3) | 108 (3) | 2 (1) | |
| II | 1395 (40) | 1349 (41) | 46 (22) | |
| III | 1555 (45) | 1440 (44) | 115 (55) | |
| IV | 402 (12) | 357 (11) | 45 (22) | |
| Coronary artery disease | 1428 (41) | 1333 (41) | 95 (46) | .19 |
| Previous PCI | 1056 (32) | 992 (32) | 64 (33) | .74 |
| Previous CABG | 427 (13) | 402 (13) | 25 (13) | .99 |
| Atrial fibrillation | 1163 (34) | 1075 (33) | 88 (43) | <.01 |
| Pacemaker | 393 (11) | 361 (11) | 32 (15) | .06 |
| Chronic kidney disease | 1880 (54) | 1763 (54) | 117 (56) | .58 |
| COPD | 903 (26) | 824 (25) | 79 (38) | <.01 |
| Stroke/TIA | 335 (10) | 310 (10) | 25 (13) | .18 |
| Peripheral artery disease | 710 (21) | 645 (21) | 65 (32) | <.01 |
| EuroSCORE II | 4.0 [2.3-7.2] | 4.0 [2.2-7.1] | 5.1 [2.6-8.7] | <.01 |
| STS-PROM score | 3.8 [2.5-5.8] | 3.8 [2.5-5.8] | 4.2 [2.8-6.7] | <.01 |
| Echocardiographic assessment | ||||
| LVEF | 55.7 (13.0) | 55.9 (12.9) | 53.3 (14.4) | <.01 |
| LVEF ≤40 | 517 (15) | 471 (15) | 46 (23) | <.01 |
| Aortic valve area, cm2 | 0.7 [0.6-0.8] | 0.7 [0.6-0.8] | 0.7 [0.6-0.8] | .96 |
| Aortic peak gradient, mmHg | 76.2 (24.5) | 76.7 (24.4) | 68.5 (24.1) | <.01 |
| Aortic mean gradient, mmHg | 46.4 (16.0) | 46.7 (15.9) | 41.7 (15.6) | <.01 |
| Calcium score, Agatston | 2277[1421-3321] | 2316[1493-3341] | 1611[1147-2620] | <.01 |
| Low-flow low-gradient aortic stenosis | 1057 (31) | 972 (30) | 82 (42) | <.01 |
CABG, coronary artery bypass grafting; COPD, chronic pulmonary obstructive disease; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
At 1 month of follow-up, 208 patients (6%) were classified as NYHA class III-IV. Of them, 160 (77%) showed advanced NYHA class (III or IV) before TAVR (4 of them required hospitalization due to HF within the first month after TAVR); 31 (15%) showed a deterioration in their clinical status without HF hospitalization in the first month; and 17 (8%) showed a decline with HF hospitalization within the first month of follow-up.
Patients with worse functional class after TAVR were similar in age and sex distribution to those in NYHA class I-II. However, patients in NYHA class III-IV showed a higher burden of comorbidities such as hypertension (93% vs 85%, P<.01), atrial fibrillation (43% vs 33%, P<.01), and COPD (38% vs 25%, P<.01). Consistently, these patients had a worse risk profile according to STS score (4.2 [2.8 -6.7]% vs 3.8 [2.5-5.8]%; P<.01) and EuroSCORE II (5.1 [2.6-8.7]% vs 4.0 [2.2-7.1]%; P<.01).
NYHA class III-IV patients had lower left ventricular ejection fraction (LVEF) (53.3±14.4% vs 55.9±12.9%; P<.01), with a higher proportion of patients with reduced LVEF (LVEF ≤ 40%: 23% vs 15%; P<.01), and lower peak and mean transaortic gradients (peak: 68.5±24.1 mmHg vs 76.7±24.4 mmHg; P<.01; mean: 41.7±15.6 mmHg vs 46.7±15.9mmHg; P<.01). Consequently, the rate of low-gradient AS was higher among patients with NYHA class III-IV at 30-day follow-up (42% vs 30%; P<.01).
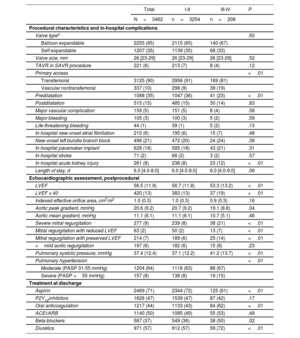
Procedural characteristics and early outcomesTable 2 summarizes the procedural-related characteristics, in-hospital complications, and postprocedural echocardiogram results.
Procedural-related characteristics, echocardiographic assessment, and treatment at discharge
| Total | I-II | III-IV | P | |
|---|---|---|---|---|
| N=3462 | n=3254 | n=208 | ||
| Procedural characteristics and in-hospital complications | ||||
| Valve typea | .50 | |||
| Balloon-expandable | 2255 (65) | 2115 (65) | 140 (67) | |
| Self-expandable | 1207 (35) | 1139 (35) | 68 (33) | |
| Valve size, mm | 26 [23-29] | 26 [23-29] | 26 [23-29] | .52 |
| TAVR in SAVR procedure | 221 (6) | 213 (7) | 8 (4) | .12 |
| Primary access | <.01 | |||
| Transfemoral | 3125 (90) | 2956 (91) | 169 (81) | |
| Vascular nontransfemoral | 337 (10) | 298 (9) | 39 (19) | |
| Predilatation | 1088 (35) | 1047 (36) | 41 (23) | <.01 |
| Postdilatation | 515 (15) | 485 (15) | 30 (14) | .83 |
| Major vascular complication | 159 (5) | 151 (5) | 8 (4) | .58 |
| Major bleeding | 105 (3) | 100 (3) | 5 (2) | .59 |
| Life-threatening bleeding | 44 (1) | 39 (1) | 5 (2) | .13 |
| In-hospital new-onset atrial fibrillation | 210 (6) | 195 (6) | 15 (7) | .48 |
| New-onset left bundle branch block | 496 (21) | 472 (20) | 24 (24) | .39 |
| In-hospital pacemaker implant | 628 (18) | 585 (18) | 43 (21) | .31 |
| In-hospital stroke | 71 (2) | 68 (2) | 3 (2) | .57 |
| In-hospital acute kidney injury | 261 (8) | 238 (8) | 23 (12) | <.01 |
| Length of stay, d | 6.0 [4.0-8.0] | 6.0 [4.0-8.0] | 6.0 [4.0-9.0] | .06 |
| Echocardiographic assessment, postprocedural | ||||
| LVEF | 56.5 (11.9) | 56.7 (11.8) | 53.3 (13.2) | <.01 |
| LVEF ≤ 40 | 420 (13) | 383 (13) | 37 (19) | <.01 |
| Indexed effective orifice area, cm2/m2 | 1.0 (0.3) | 1.0 (0.3) | 0.9 (0.3) | .16 |
| Aortic peak gradient, mmHg | 20.6 (9.2) | 20.7 (9.2) | 19.1 (8.8) | .04 |
| Aortic mean gradient, mmHg | 11.1 (6.1) | 11.1 (6.1) | 10.7 (5.1) | .46 |
| Severe mitral regurgitation | 277 (9) | 239 (8) | 38 (21) | <.01 |
| Mitral regurgitation with reduced LVEF | 63 (2) | 50 (2) | 13 (7) | <.01 |
| Mitral regurgitation with preserved LVEF | 214 (7) | 189 (6) | 25 (14) | <.01 |
| >mild aortic regurgitation | 197 (6) | 182 (6) | 15 (8) | .23 |
| Pulmonary systolic pressure, mmHg | 37.4 (12.4) | 37.1 (12.2) | 41.2 (13.7) | <.01 |
| Pulmonary hypertension | <.01 | |||
| Moderate (PASP 31-55 mmHg) | 1204 (64) | 1116 (63) | 88 (67) | |
| Severe (PASP >55 mmHg) | 157 (8) | 138 (8) | 19 (15) | |
| Treatment at discharge | ||||
| Aspirin | 2469 (71) | 2344 (72) | 125 (61) | <.01 |
| P2Y12inhibitors | 1626 (47) | 1539 (47) | 87 (42) | .17 |
| Oral anticoagulation | 1217 (44) | 1133 (43) | 84 (62) | <.01 |
| ACEi/ARB | 1140 (50) | 1085 (49) | 55 (53) | .49 |
| Beta-blockers | 587 (37) | 549 (36) | 38 (50) | .02 |
| Diuretics | 971 (57) | 912 (57) | 59 (72) | <.01 |
ACEi, angiotensin converting enzyme inhibitors; ARB, angiotensin receptors blockers; LVEF, left ventricular ejection fraction; PASP, pulmonary artery systolic pressure; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
The transfemoral approach was used in 90% of patients, with a higher rate of a nontransfemoral approach in patients with 1-month NYHA class III-IV (19% vs 9%; P<.01). NYHA class III-IV patients less often had valve predilatation during the procedure (23% vs 36%; P<.01). The groups did not differ in any other procedural-related characteristic or the type and size of the implanted valve. The rate of procedural and in-hospital complications was similar between the 2 groups.
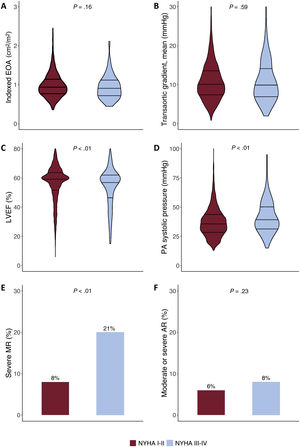
In the postprocedural echocardiogram, the mean indexed effective orifice area and mean aortic gradient were 1.0±0.3 cm2/m2 and 11.1±6.1 mmHg, respectively, with no differences between groups. Conversely, patients with 1-month NYHA class III-IV had a lower LVEF and a higher rate of LVEF ≤40% (19% vs 13%; P<.01); a higher rate of severe mitral regurgitation (21% vs 8%; P<.01); higher pulmonary artery systolic pressure (41.2±13.7 mmHg vs 37.1±12.2 mmHg; P≤.01); and a higher rate of severe pulmonary hypertension (15% vs 8%; P<.01) (figure 2, table 2).
Comparison of echocardiographic parameters after procedure between patients showing NYHA class I-II vs NYHA class III-IV at 1 month of follow -up. A: indexed effective orifice area. B: transaortic mean gradient. C: left ventricular ejection fraction. D: pulmonary artery systolic pressure. E: severe mitral regurgitation. F: moderate or severe aortic regurgitation. AR, aortic regurgitation; iEOA, indexed effective orifice area; LVEF, left ventricle ejection fraction; MR, mitral regurgitation; PA, pulmonary artery.
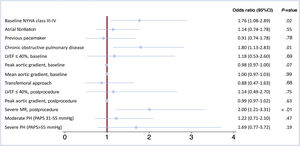
The results of the univariable logistic regression analysis are described in the table 2 of the supplementary data. Figure 3 shows the results of the multivariable analysis performed to determine the predictors of 1-month impaired functional status. In the multivariable analysis, NYHA III-IV before TAVR was associated with a higher risk of having advanced NYHA class at 1-month follow-up (OR, 1.76; 95%CI, 1.08-2.89; P=.02). A history of COPD (OR, 1.80; 95%CI, 1.13-2.83; P=.01) and the presence of severe mitral regurgitation in the echo performed after the procedure (OR, 2.00; 95%CI, 1.21-3.31; P<.01) were also independent predictors of impaired NYHA class at 1-month follow-up.
Multivariable logistic regression analysis representing the association of different covariates with the presence of NYHA class III-IV 1 month after TAVR. 95%CI, 95% confidence interval; LVEF, left ventricle ejection fraction; MR, mitral regurgitation; NYHA, New York Heart Association; PAPS, pulmonary artery pressure, systolic; PH, pulmonary hypertension.
A subanalysis of the factors associated with NYHA class III-IV at the 30-day follow-up was performed including only patients without previous COPD. In this subpopulation, postprocedural severe mitral regurgitation (OR, 2.24; 95%CI, 1.22-4.10); P<.01) and new-onset atrial fibrillation (OR, 3.08; 95%CI; 1.38-6.87; P<.01) independently predicted the presence of NYHA class III-IV at 30 days, while baseline NYHA class III-IV was no longer a predictor of 30-day NYHA class III-IV (table 3 of the supplementary data).
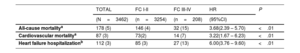
Prognostic implications of advanced NYHA classThe median follow-up of the population was 12 [4-24] months. In 188 patients (5% of the final population), the follow-up was missing after the 1-month visit. During the first year after TAVR, 178 patients (5%) died, and 112 (3%) required rehospitalization due to an HF episode (table 3).
Comparison of event occurrence and risk for events at 1 year according to 1-month NYHA class
| TOTAL | FC I-II | FC III-IV | HR | P | |
|---|---|---|---|---|---|
| (N=3462) | (n=3254) | (n=208) | (95%CI) | ||
| All-cause mortalitya | 178 (5) | 146 (4) | 32 (15) | 3.68(2.39 – 5.70) | <.01 |
| Cardiovascular mortalitya | 87 (3) | 73(2) | 14 (7) | 3.22(1.67 – 6.23) | <.01 |
| Heart failure hospitalizationb | 112 (3) | 85 (3) | 27 (13) | 6.00(3.76 – 9.60) | <.01 |
95%CI, 95% confidence interval; COPD, chronic pulmonary obstructive disease; FC, functional class; HR, hazard ratio; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class; Unless otherwise indicated, the data are expressed as No. (%).
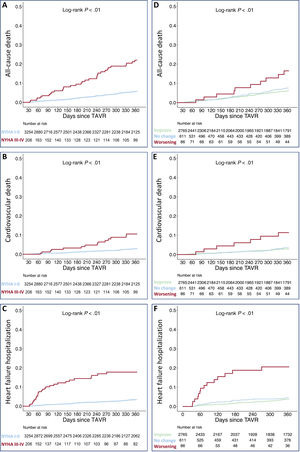
The presence of advanced NYHA class at 1-month follow-up was associated with a higher risk of all-cause mortality (HR, 3.68; 95%CI, 2.39-5.70; P<.01), cardiovascular mortality (HR, 3.22; 95%CI, 1.67-6.23; P<.01), and HF-related hospitalization (HR, 6.00; 95%CI, 3.76-9.60; P<.01).
In addition, worsening functional class (from baseline to 1-month post-TAVR) was associated with a higher risk of all-cause mortality (HR, 2.87; 95%CI, 1.50-5.53; P<.01), cardiovascular mortality (HR, 4.48; 95%CI, 2.02-9.96; P<.01), and HF hospitalization (HR, 6.38; 95%CI, 3.48-11.7; P<.01). Figure 4 shows a comparison of survival curves between patients with 1-month NYHA class I-II and III-IV; and between patients with worsening of their functional class and those who remained equal or improved.
Kaplan-Meier estimates of 1-year all-cause mortality (A) cardiovascular mortality (B) and heart failure hospitalization (C) according to the presence of NYHA class III-IV at 1 month of follow-up. Kaplan-Meier estimates of 1-year all-cause mortality (D) cardiovascular mortality (E) and heart failure hospitalization (F) according to the change in NYHA functional class from baseline to 30 days of follow-up. TAVR, transcatheter aortic valve replacement.
In this large multicenter series of patients undergoing TAVR with newer generation TAVR devices, 208 patients (6%) showed NYHA class III-IV 1 month after the procedure. Predictors of NYHA class III-IV at 1 month of follow-up were NYHA class III-IV before TAVR, COPD, and severe mitral regurgitation on the postprocedural echocardiogram. The presence of an impaired functional status 1 month after TAVR led to a 3- and 6-fold increased risk of mortality and HF hospitalization, respectively, at 1-year follow-up (figure 5).
Central illustration. Prevalence, predictors and prognostic significance of impaired functional status following transcatheter aortic valve replacement. 95%CI, 95% confidence interval; AS, aortic stenosis; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; IQR, interquartile range; MR, mitral regurgitation; NYHA, New York Heart Association; OR, odds ratio; TAVR, transcatheter aortic valve replacement.
Although TAVR has been consistently shown to improve NYHA class, a lack of improvement 1 month after TAVR has been reported in up to 16% of patients.7 In addition, persistent NYHA class III-IV at 1-year follow-up or HF hospitalization within the first year after TAVR was reported in about 7% of patients included in a multicenter Japanese registry.6 In our population, 20% of patients showed no improvement in NYHA class, and 6% were classified as NYHA class III or IV 1 month after the procedure.
Although TAVR has been demonstrated to be a cost-effective therapy in intermediate- and high-risk populations,9 these data raise concerns about the futility of TAVR in some patients, especially those with a higher comorbidity burden.8 Indeed, previous studies showed that up to one-third of patients have poor quality of life or die within the first year after TAVR. In addition, functional decline or lack of improvement is common in frail patients undergoing TAVR.10
COPD was an independent predictor of NYHA class III-IV at 1 month post-TAVR. COPD patients constitute a high-risk population, with an increased risk of respiratory-related complications after cardiac surgery.11 COPD patients undergoing TAVR have fewer respiratory complications and lower in-hospital mortality than those undergoing surgery.11 However, COPD is related to higher 1-year mortality and is more frequent in patients with no improvement of NYHA class in the first month after TAVR.6,7,12 Evaluating dyspnea in patients with COPD and severe AS may be challenging. The severity of intrinsic pulmonary disease may hamper the improvement of shortness of breath in COPD patients. Therefore, a correct assessment of pulmonary disease and its optimal treatment is desirable in these patients to improve patient-perceived outcomes. Regardless, patients with COPD should be informed about the possibility of a mild or no improvement in dyspnea symptoms after the procedure. A detailed explanation of the risk/benefit ratio of the intervention should be given, focusing on the potential survival improvement resulting from the procedure, even in the absence of significant symptomatic relief.
Interestingly, when we analyzed patients without COPD, the baseline NYHA class did not show a significant association with the presence of NYHA class III-IV at the 30-day follow-up. This finding highlights the impact of pulmonary disease on the symptoms of patients with valvular heart disease and the need for a systematic approach to patients’ comorbidities in order to improve TAVR outcomes.
Earlier intervention in aortic stenosisPatients with impaired NYHA class 1 month after TAVR showed several signs of more advanced cardiac disease before the procedure, including higher rates of NYHA class III-IV and atrial fibrillation, and lower LVEF. In addition, these patients more frequently had low-flow low-gradient severe AS.
Patients undergoing TAVR in NYHA class III-IV have a worse clinical course, with a higher risk of mortality and HF hospitalization during the first year of follow-up.13,14 In addition, nonreversible pulmonary hypertension is related to poorer outcomes.15
The onset of AS-related symptoms is often accompanied by other cardiac injury,15 reducing the effectiveness of the aortic valve replacement in symptom relief and survival improvement.15–17
In the AVATAR trial, early surgery in asymptomatic AS patients reduced the composite outcome of all-cause death, acute myocardial infarction, stroke, or unplanned HF hospitalization.18 All this evidence would favor an early intervention in patients with severe AS before nonreversible cardiac injury appears, hampering the patient's symptom relief and survival. However, this enhanced proactive strategy would unavoidably lead to complications among asymptomatic patients and a substantial increase in health care expenditure. Thus, it is important to identify early markers of cardiac injury to improve the selection of asymptomatic patients who might benefit from early intervention. In addition, the ongoing EARLY-TAVR trial (NCT03042104) evaluating the effectiveness of TAVR (vs medical treatment) in reducing the composite endpoint of all-cause death, stroke, and unplanned cardiovascular hospitalization in asymptomatic AS patients will provide a definite response regarding the timing of the intervention. Meanwhile, once severe AS is diagnosed, patients should be closely followed up and undergo early intervention when symptoms appear, avoiding a delayed intervention at later stages of the disease.
Combined mitral-aortic diseaseBaseline moderate-to-severe MR is present in about one-fifth of patients undergoing TAVR and is associated with increased mortality. TAVR reduces the severity of MR in up to 60% of patients. However, residual MR after TAVR is associated with increased 1-year mortality.19
Data from the TRAMI registry showed that almost 9% of patients undergoing mitral transcatheter edge-to-edge repair (TEER) had a previous aortic valve replacement, either surgical or TAVR. Notably, patients with previous aortic valve replacement were older and had a higher rate of COPD, and 87% of them were in NYHA class III-IV at the time of TEER. Patients undergoing TEER after TAVR had an estimated 1-year survival below 50%.20 This study did not report the time from TAVR to TEER, so late interventions cannot be excluded. Of all the patients registered for TAVR, TEER, or transcutaneous mitral valve replacement at the United States Readmissions Database from 2014 to 2018, 110 patients underwent simultaneous TAVR and TEER. Simultaneous TAVR and TEER was associated with poorer outcomes compared with TAVR alone, reflecting the potential risk of a simultaneous procedure in an elderly population with a high comorbidity burden.21 In a multicenter study of TAVR patients with postprocedural moderate-to-severe MR and persistence of NYHA class III-IV in 90%, TEER at a median time of 164 days after TAVR was associated with significant improvement in MR and NYHA class. In addition, a trend toward improved survival was observed.22 Our data support the concept of the high risk associated with residual MR leading to impaired functional status early after TAVR. In addition, the increased mortality risk related to significant residual MR seems to start very early after the procedure, highlighting the major clinical relevance of close follow-up, medical treatment optimization, and earlier mitral intervention in such cases. The ongoing MITAVI (Treatment of Concomitant Mitral Regurgitation by Mitral Valve Clipping in Patients With Successful Transcatheter Aortic Valve Implantation) randomized trial (NCT04009434), assessing whether TAVR patients with moderate-to-severe MR can benefit from additional mitral valve clipping treatment, will provide important insights regarding the timing of intervention.
Early evaluation of patients after transcatheter aortic valve replacementThe adverse prognostic impact of advanced NYHA class at baseline or 1 year after TAVR has been previously described.6,12,13
In our population, NYHA class III-IV or an episode of HF hospitalization 1 month after TAVR was related to an approximately 3-fold higher mortality and up to 6-fold increased risk for HF hospitalization within the year following TAVR. Furthermore, most events occurred during the first 6 months of follow-up. This means that delaying interventions in these patients may significantly affect their prognosis.
We identified several factors at hospital discharge predicting advanced NYHA class at 1 month of follow-up. Besides an earlier intervention of patients requiring TAVR, an earlier evaluation of patients after TAVR is advisable to identify those with suboptimal symptoms improvement and detect possible causes for this outcome. Treatment should be optimized to improve comorbidity-related symptoms and prognosis (diuretic regimen, HF treatment, COPD treatment). The involvement of HF specialists should also be strongly considered in such cases. In addition, in patients with multivalvular disease, a planned staged procedure might help to improve symptom relief and prognosis. Further studies are necessary to address this topic.
Study limitationsThis study has several limitations. This was a nonprespecified retrospective analysis of prospectively collected data. Consequently, some information relevant to the study topic may have not been consistently collected. Information on NYHA class at 30 days was missing in 18% of patients screened for inclusion, partially due to the loss of follow-up visits during the coronavirus pandemic from 2020 to 2021. The NYHA functional classification is semiquantitative, and its subjective assessment might be subject to interobserver variability and influenced by patients’ comorbidities and frailty. NYHA class was graded according to investigator criteria based on clinical interview but not based on standardized questionnaires. Due to its observational nature, the presence of unmeasured confounders that can influence study conclusions cannot be excluded. Detailed information on some echocardiographic signs of cardiac injury was not available. In addition, information on the completeness of the coronary revascularization before TAVR was available for less than one-third of the patients. Finally, specific information on COPD severity and the need for home oxygen therapy was not available.
CONCLUSIONSNYHA class III-IV at 1 month after transarterial TAVR was present in 6% of patients and was related to a worse baseline functional status, the presence of pulmonary disease, and significant residual MR following the procedure. Impaired functional status early after TAVR conferred a major increased risk of poorer outcomes, including death and HF hospitalization events. These results would suggest the advisability of earlier intervention strategies for AS patients to avoid advanced symptoms, along with a close follow-up and a highly proactive medical and interventional strategy among those with residual MR. Further studies on treatment optimization in patients with suboptimal functional status early after TAVR are urgently warranted.
FUNDINGJ. Nuche is the recipient of a grant from the Fundación Alfonso Martín Escudero (Madrid, Spain). J. Rodés-Cabau holds the Research Chair “Fondation Famille Jacques Larivière” for the Development of Structural Heart Disease Interventions (Laval University).
ETHICAL CONSIDERATIONSThis study was conducted according to the ethics committee of each participating center, and all patients provided signed informed consent for the procedures. This study was conducted in accordance with the SAGER (Sex and Gender Equity in Research) guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence tools have been used for the preparation of this study.
AUTHORS’ CONTRIBUTIONSJ. Nuche and J. Rodés-Cabau conceived and designed the study. J. Nuche and J. Mesnier merged local databases from all participating centers and were responsible for quality of data control. J. Nuche performed the statistical analysis and wrote the first draft of the manuscript. All authors participated in the design and completion of local databases. All authors approved the final version of the manuscript and ensured the accuracy and integrity of the work. All authors had access to all the data in the study and had final responsibility for the decision to submit the manuscript for publication. J. Rodés-Cabau is responsible for the overall content of the study as guarantor.
CONFLICTS OF INTERESTJ. Ternacle is a consultant for Abbott. T. Modine is a consultant for Abbott, Edwards Lifesciences, and Medtronic. L. Asmarats has received speaker fees from Edwards Lifesciences. J. Rodés-Cabau has received institutional research grants and consultant/speaker fees from Edwards Lifesciences and Medtronic. All other authors report that they have no relationships relevant to the contents of this article to disclose.
- -
TAVR has become the standard of care for patients with severe symptomatic AS.
- -
However, a nonnegligible proportion of patients have poor quality of life or die within the first year after TAVR.
- -
Patients with NYHA class III/IV 1 month after TAVR had a higher burden of comorbidities, with COPD as an independent predictor of impaired functional outcomes.
- -
Baseline NYHA class III-IV and severe postprocedural MR also predicted NYHA class III-IV at 1 month. NYHA III-IV at 1 month was associated with a higher risk of mortality and HF hospitalization within the first year after TAVR.
- -
This study highlights the importance of an appropriate evaluation of TAVR patients not only before the procedure but also soon after discharge. Appropriate timing for the intervention and the optimal approach for multivalvular disease should be assessed in dedicated studies.