Thrombocytopenia frequently occurs after transcatheter aortic valve implantation (TAVI) but its impact is poorly understood. We aimed to analyze the incidence, clinical impact, and predictors of acquired thrombocytopenia after TAVI.
MethodsThis retrospective multicenter registry included 3913 patients undergoing TAVI with a baseline platelet count of ≥ 100 *109/L. Acquired thrombocytopenia was defined as a decrease in baseline platelet count of ≥ 50% (early nadir ≤ 3 days and late nadir ≥ 4 days) post-TAVI. The primary endpoint was 30-day all-cause mortality and secondary endpoints were procedural safety and 2-year all-cause mortality.
ResultsThe incidence of acquired thrombocytopenia was 14.8% (early nadir: 61.5%, late nadir: 38.5%). Thirty-day mortality occurred in 112 (3.0%) patients and was significantly higher in those with thrombocytopenia (8.5% vs 2.0%, adjusted OR, 2.3; 95%CI, 1.3-4.2). Procedural safety was lower and 2-year mortality was higher in patients with thrombocytopenia vs those without (52.1 vs 77.0%; P <.001, and 30.2% vs 16.8%; HR, 2.2, 95%IC, 1.3-2.7) and especially in those with late nadir thrombocytopenia (45.8% vs 54.5%; P=.056, and 38.6% vs 23.8%, HR, 2.1; 95%CI, 1.5-2.9). Independent predictors of thrombocytopenia comprised baseline and procedural factors such as body surface area, absence of diabetes, poorer renal function, peripheral vascular disease, nontransfemoral access, vascular complications, type of transcatheter heart valve, and earlier TAVI procedures.
ConclusionsAcquired thrombocytopenia was common (15%) after TAVI and was associated with increased short- and mid-term mortality and decreased procedural safety. Moreover, late thrombocytopenia compared with early thrombocytopenia was associated with significantly worse clinical outcomes. Further investigations are needed to elucidate the etiologic mechanisms behind these findings.
Keywords
Abreviations
Acute postprocedural thrombocytopenia is a common finding (∼50%) in patients undergoing cardiac surgery and is a marker of poor prognosis with increased mortality and longer hospitalization.1,2 Transcatheter aortic valve implantation (TAVI) has emerged as a minimally invasive alternative to surgery for patients with severe aortic stenosis, offering a lower risk of bleeding, shorter intensive care unit and hospital stays, and faster recovery.3 Given the less invasive nature of TAVI, with minimal blood loss and a lower inflammatory response, a smaller decrease in platelet count would be expected. However, thrombocytopenia remains a frequent complication after TAVI, occurring in up to 35% of patients, and has been associated with worse short- and mid-term clinical outcomes.4,5 Despite this, limited attention has been paid to this issue, and most evidence comes from single-center studies with small patient numbers, which could introduce bias due to specific procedural techniques or periprocedural management. The aim of this study was to analyze the incidence, timing, predictors, and clinical impact of acquired thrombocytopenia in a large multicenter cohort of patients undergoing TAVI.
METHODSStudy design, procedural details, and data collectionThis observational multicenter registry included consecutive patients with symptomatic severe aortic stenosis undergoing TAVI from 8 centers between 2008 and 2021. Patients with baseline thrombocytopenia (< 100*109/L), unavailable platelet counts (pre- or post-TAVI), or intraprocedural death were excluded from the analysis. Eligibility for TAVI and postprocedural management was determined by each Heart Team at the individual center. The decision regarding the type and size of the transcatheter heart valve (THV), access route, and implantation technique was at the discretion of the TAVI operator. Intraprocedural anticoagulation was achieved with unfractionated heparin boluses, starting at 70-100 U/kg at the beginning of the procedure and adjusted to maintain an activated clotting time of >250seconds. Periprocedural antiplatelet therapy was administered according to the protocol in each center. Baseline characteristics, procedural details, clinical outcomes, and follow-up data were prospectively collected in a dedicated centralized database at the coordinating center. Platelet count data were retrospectively collected from laboratory results available in electronic medical records. All patients provided informed consent before the procedure, and the study was conducted in accordance with the institutional review board requirements of the participating centers. This research was undertaken without direct patient involvement; patients were not invited to comment on the study design, develop patient-relevant outcomes, or interpret the results. There was no dedicated funding for this study.
DefinitionsPostprocedural thrombocytopenia was defined as a ≥ 50% decrease in platelet count compared with baseline within 0 to 10 days after TAVI.6 The percentage decrease in platelet count (DPC) was calculated as: [(baseline platelet count - nadir platelet count)/baseline platelet count]*100]. Early platelet count nadir was defined as the lowest platelet count within 3 days post-TAVI, and late nadir was defined as occurring from day 4 onward.4 Clinical outcomes were defined according to the Valve Academic Research Consortium (VARC-2) criteria. To determine the presence of prosthesis patient mismatch, previously defined predicted effective orifice area for each valve type and size were used, and indexed by body surface area.7,8 The primary endpoint was 30-day all-cause mortality, and the secondary endpoints were procedural safety and 2-year all-cause mortality.
Statistical analysisThe data are reported as mean and standard deviation for continuous variables and as numbers and percentages (%) for categorical variables. All patients were classified according to the presence or absence of postprocedural thrombocytopenia, and comparison were performed using the 2-sided Student t test for continuous variables and the chi-square test for categorical variables, as appropriate.
A subanalysis was conducted to assess the impact of platelet count dynamics. Patients were classified into 3 groups: group 1, without thrombocytopenia; group 2, thrombocytopenia with early nadir; and group 3, thrombocytopenia with late nadir. To avoid selection bias, only patients with a hospital stay of ≥ 4 days (and with available hemogram beyond day 3) and those with an increase in platelet count after the post-TAVI nadir were included in this subanalysis.
Survival analysis was performed using a Kaplan-Meier survival function and the curves were compared using the log-rank test. Logistic regression was used to assess predictors of thrombocytopenia and 30-day mortality. The variables associated with these outcomes in the univariable analysis (with a P value of <.100) were included in the multivariable logistic model. In addition, a propensity score matching analysis was performed to obtain 2 matched groups with and without thrombocytopenia, adjusted for baseline and procedural characteristics. The primary and secondary endpoints were analyzed in this matched cohort to assess the clinical impact of thrombocytopenia. Details of the propensity score matching method are provided in the .
A subgroup analysis was performed to evaluate the impact of thrombocytopenia on primary and secondary endpoints in subgroups defined by sex (male and female), access type (transfemoral and nontransfemoral), different THV types, and TAVI procedures performed before and after 2015. The year 2015 was chosen as it represented the mid-point of the inclusion period. Due to advances in technique and data from large, randomized studies, patients from 2015 onwards represent a population more comparable to current clinical practice in TAVI, which was the rationale for this subgroup analysis.
P values of less than .05 were considered statistically significant. All data were analyzed using Stata 14 (StataCorp, College Station, United States).
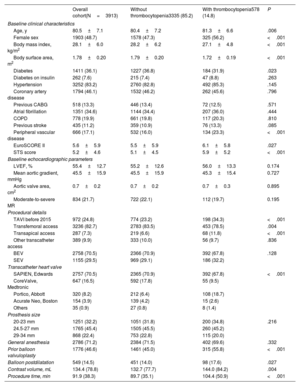
RESULTSAmong 4580 patients undergoing TAVI, a total of 3913 patients were considered eligible for the study. The main reasons for exclusion were baseline thrombocytopenia (< 100*109/L) in 128 patients, absence of baseline platelet count in 503 patients, and absence of postprocedural platelet count in 36 patients. Baseline and procedural characteristics of the entire cohort, as well as according to acquired thrombocytopenia, are presented in table 1. The mean age was 80.5±7.1 years, and the mean Society of Thoracic Surgeons (STS) risk of mortality was 5.2%±4.6. Transfemoral access was used in 3236 (82.7%) patients. Patients with acquired thrombocytopenia were slightly older, more likely to be female, had a lower body surface area, higher surgical risk scores, and were more likely to have received a transapical approach (table 1).
Baseline characteristics and procedural details
| Overall cohort(N=3913) | Without thrombocytopenia3335 (85.2) | With thrombocytopenia578 (14.8) | P | |
|---|---|---|---|---|
| Baseline clinical characteristics | ||||
| Age, y | 80.5±7.1 | 80.4±7.2 | 81.3±6.6 | .006 |
| Female sex | 1903 (48.7) | 1578 (47.3) | 325 (56.2) | <.001 |
| Body mass index, kg/m2 | 28.1±6.0 | 28.2±6.2 | 27.1±4.8 | <.001 |
| Body surface area, m2 | 1.78±0.20 | 1.79±0.20 | 1.72±0.19 | <.001 |
| Diabetes | 1411 (36.1) | 1227 (36.8) | 184 (31.9) | .023 |
| Diabetes on insulin | 262 (7.6) | 215 (7.4) | 47 (8.8) | .263 |
| Hypertension | 3252 (83.2) | 2760 (82.8) | 492 (85.3) | .145 |
| Coronary artery disease | 1794 (46.1) | 1532 (46.2) | 262 (45.6) | .796 |
| Previous CABG | 518 (13.3) | 446 (13.4) | 72 (12.5) | .571 |
| Atrial fibrillation | 1351 (34.6) | 1144 (34.4) | 207 (36.0) | .444 |
| COPD | 778 (19.9) | 661 (19.8) | 117 (20.3) | .810 |
| Previous stroke | 435 (11.2) | 359 (10.9) | 76 (13.3) | .085 |
| Peripheral vascular disease | 666 (17.1) | 532 (16.0) | 134 (23.3) | <.001 |
| EuroSCORE II | 5.6±5.9 | 5.5±5.9 | 6.1±5.8 | .027 |
| STS score | 5.2±4.6 | 5.1±4.5 | 5.9±5.2 | <.001 |
| Baseline echocardiographic parameters | ||||
| LVEF, % | 55.4±12.7 | 55.2±12.6 | 56.0±13.3 | 0.174 |
| Mean aortic gradient, mmHg | 45.5±15.9 | 45.5±15.9 | 45.3±15.4 | 0.727 |
| Aortic valve area, cm2 | 0.7±0.2 | 0.7±0.2 | 0.7±0.3 | 0.895 |
| Moderate-to-severe MR | 834 (21.7) | 722 (22.1) | 112 (19.7) | 0.195 |
| Procedural details | ||||
| TAVI before 2015 | 972 (24.8) | 774 (23.2) | 198 (34.3) | <.001 |
| Transfemoral access | 3236 (82.7) | 2783 (83.5) | 453 (78.5) | .004 |
| Transapical access | 287 (7.3) | 219 (6.6) | 68 (11.8) | <.001 |
| Other transcatheter access | 389 (9.9) | 333 (10.0) | 56 (9.7) | .836 |
| BEV | 2758 (70.5) | 2366 (70.9) | 392 (67.8) | .128 |
| SEV | 1155 (29.5) | 969 (29.1) | 186 (32.2) | |
| Transcatheter heart valve | ||||
| SAPIEN, Edwards | 2757 (70.5) | 2365 (70.9) | 392 (67.8) | <.001 |
| CoreValve, Medtronic | 647 (16.5) | 592 (17.8) | 55 (9.5) | |
| Portico, Abbott | 320 (8.2) | 212 (6.4) | 108 (18.7) | |
| Acurate Neo, Boston | 154 (3.9) | 139 (4.2) | 15 (2.6) | |
| Others | 35 (0.9) | 27 (0.8) | 8 (1.4) | |
| Prosthesis size | ||||
| 20-23 mm | 1251 (32.2) | 1051 (31.8) | 200 (34.8) | .216 |
| 24.5-27 mm | 1765 (45.4) | 1505 (45.5) | 260 (45.2) | |
| 29-34 mm | 868 (22.4) | 753 (22.8) | 115 (20.0) | |
| General anesthesia | 2786 (71.2) | 2384 (71.5) | 402 (69.6) | .332 |
| Prior balloon valvuloplasty | 1776 (46.6) | 1461 (45.0) | 315 (55.8) | <.001 |
| Balloon postdilatation | 549 (14.5) | 451 (14.0) | 98 (17.6) | .027 |
| Contrast volume, mL | 134.4 (78.8) | 132.7 (77.7) | 144.0 (84.2) | .004 |
| Procedure time, min | 91.9 (38.3) | 89.7 (35.1) | 104.4 (50.9) | <.001 |
BEV, balloon-expandable valve; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; LVEF, Left ventricular ejection fraction; MR, mitral regurgitation; SEV, self-expandable valve; STS, Society of Thoracic Surgeons.
Data are expressed as No. (%) or mean±standard deviation.
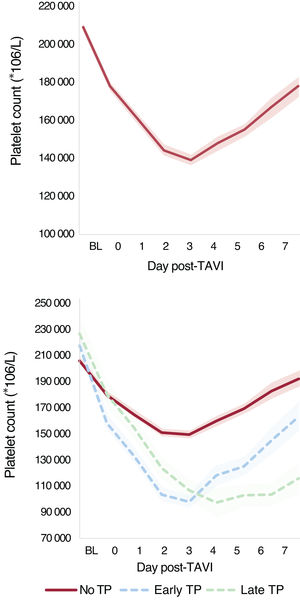
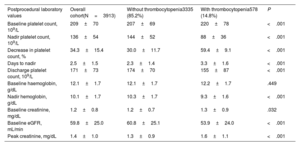
Laboratory findings are presented in table 2. The mean baseline platelet count was 209±70 *109/L and the mean post-TAVI nadir was 136±54 *109/L, occurring at 2.5±1.5 days postprocedure. The percentage DPC was 34.3%±15.4. Postprocedural thrombocytopenia (a decrease of ≥ 50% from baseline) occurred in 578 (14.8%) patients. A subsequent increase in platelet count was observed in 2461 (63.0%) patients after 4.2±1.8 days post-TAVI. The increase in platelet count was observed in 78.8% of patients with a hospital stay of ≥ 4 days. The mean platelet count at discharge was 171±73 *109/L, and the percentage decrease in platelet count at discharge relative to baseline was 16.4%±29.0. Only 19.6% of patients reached a platelet count equal to or higher than baseline values.
Postprocedural laboratory values
| Postprocedural laboratory values | Overall cohort(N=3913) | Without thrombocytopenia3335 (85.2%) | With thrombocytopenia578 (14.8%) | P |
|---|---|---|---|---|
| Baseline platelet count, 109/L | 209±70 | 207±69 | 220±78 | <.001 |
| Nadir platelet count, 109/L | 136±54 | 144±52 | 88±36 | <.001 |
| Decrease in platelet count, % | 34.3±15.4 | 30.0±11.7 | 59.4±9.1 | <.001 |
| Days to nadir | 2.5±1.5 | 2.3±1.4 | 3.3±1.6 | <.001 |
| Discharge platelet count, 109/L | 171±73 | 174±70 | 155±87 | <.001 |
| Baseline haemoglobin, g/dL | 12.1±1.7 | 12.1±1.7 | 12.2±1.7 | .449 |
| Nadir hemoglobin, g/dL | 10.1±1.7 | 10.3±1.7 | 9.3±1.6 | <.001 |
| Baseline creatinine, mg/dL | 1.2±0.8 | 1.2±0.7 | 1.3±0.9 | .032 |
| Baseline eGFR, mL/min | 59.8±25.0 | 60.8±25.1 | 53.9±24.0 | <.001 |
| Peak creatinine, mg/dL | 1.4±1.0 | 1.3±0.9 | 1.6±1.1 | <.001 |
eGFR, estimated glomerular filtration rate.
Data are expressed as mean±standard deviation.
A total of 3041 patients met the criteria of ≥ 4 days hospital stay (n=2853) or a postnadir increase in platelet count within hospital stay <4 days (n=188). In this population, early thrombocytopenia occurred in 323 (62.7%) patients, and late nadir occurred in 192 (37.3%). Platelet count dynamics are presented in figure 1 and figure 2. Platelet count at 3 months postprocedure was obtained in 2049 (54.9%) patients, with a mean platelet count of 200±73 *109/L. Thrombocytopenia was observed in only 41 (2%) patients at this follow-up. Among patients with postprocedural thrombocytopenia, only 14 (4.7%) showed persistent thrombocytopenia at follow-up.
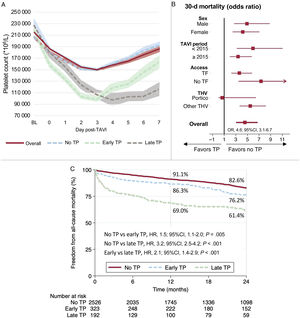
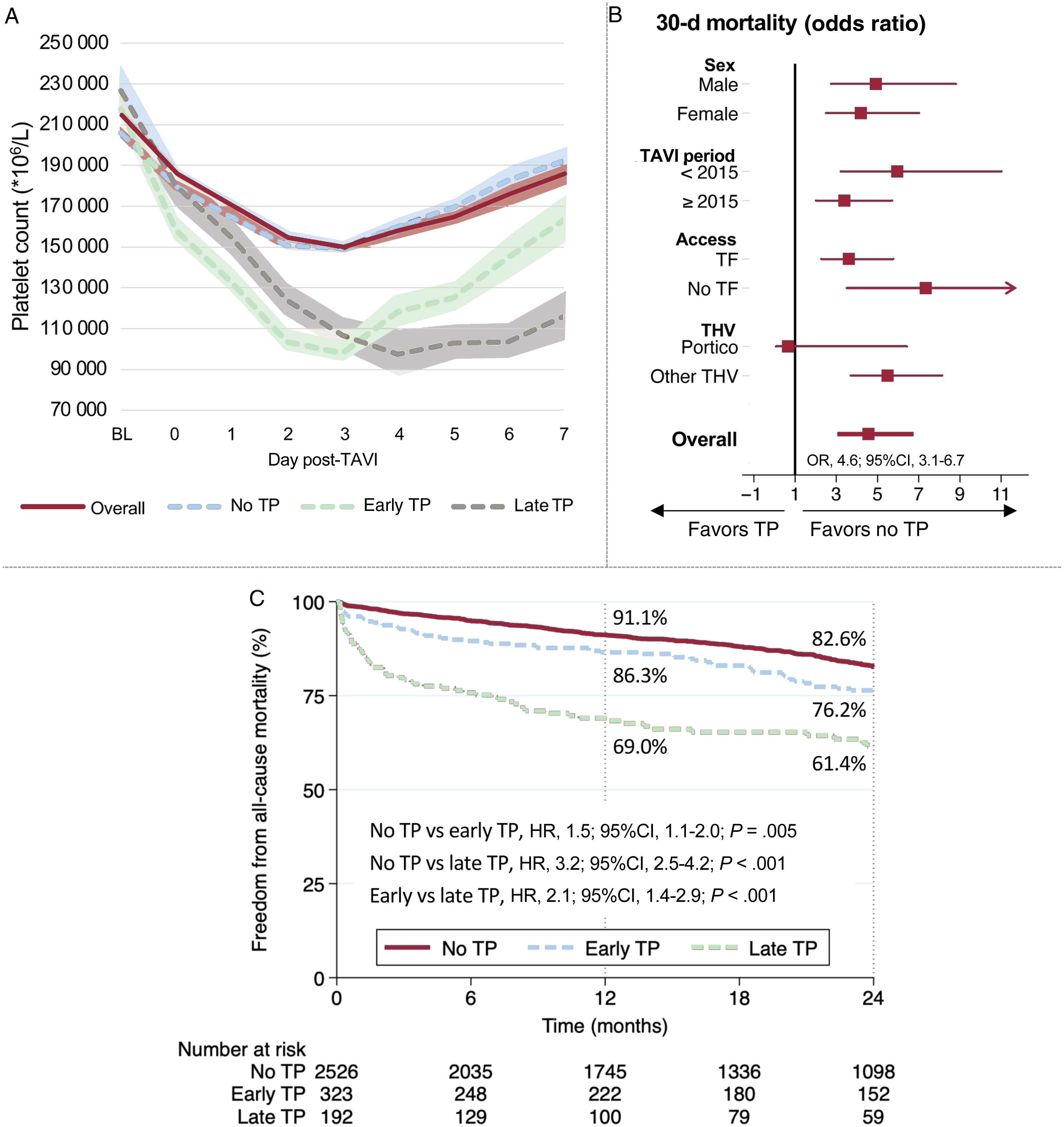
Central illustration. Platelet count pattern following TAVI and clinical impact of acquired thrombocytopenia. A: dynamics of platelet count within 7 days post-TAVI overall and according to early and late nadir. B: impact of thrombocytopenia on 30-day mortality by subgroups. C: 2-year all-cause mortality according to early and late thrombocytopenia. TF: transfemoral. THV: transcatheter heart valve. TP, thrombocytopenia.
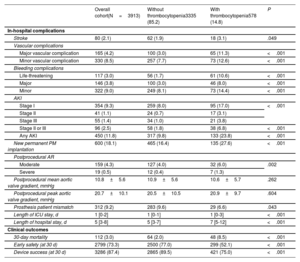
Table 3 show periprocedural complications and clinical outcomes related to the occurrence of thrombocytopenia. Overall 30-day mortality was higher in patients with thrombocytopenia (2.0 vs 8.5%; odds ratio [OR] 4.6; 95% confidence interval [95%CI], 3.1-6.7; P <.001). Other variables associated with 30-day mortality were age, STS score, major vascular complications, major and life-threatening bleeding, acute kidney injury, periprocedural stroke, and severe aortic regurgitation. The adjusted OR for 30-day mortality associated with thrombocytopenia was 2.3 (95%CI, 1.3-4.2; P=.004, ). Early safety and device success at 30 days were significantly higher in the group without thrombocytopenia (77% vs 52.1%; P <.001, and 89.5% vs 75%; P <.001, respectively).
In-hospital complications and clinical outcomes
| Overall cohort(N=3913) | Without thrombocytopenia3335 (85.2) | With thrombocytopenia578 (14.8) | P | |
|---|---|---|---|---|
| In-hospital complications | ||||
| Stroke | 80 (2.1) | 62 (1.9) | 18 (3.1) | .049 |
| Vascular complications | ||||
| Major vascular complication | 165 (4.2) | 100 (3.0) | 65 (11.3) | <.001 |
| Minor vascular complication | 330 (8.5) | 257 (7.7) | 73 (12.6) | <.001 |
| Bleeding complications | ||||
| Life-threatening | 117 (3.0) | 56 (1.7) | 61 (10.6) | <.001 |
| Major | 146 (3.8) | 100 (3.0) | 46 (8.0) | <.001 |
| Minor | 322 (9.0) | 249 (8.1) | 73 (14.4) | <.001 |
| AKI | ||||
| Stage I | 354 (9.3) | 259 (8.0) | 95 (17.0) | <.001 |
| Stage II | 41 (1.1) | 24 (0.7) | 17 (3.1) | |
| Stage III | 55 (1.4) | 34 (1.0) | 21 (3.8) | |
| Stage II or III | 96 (2.5) | 58 (1.8) | 38 (6.8) | <.001 |
| Any AKI | 450 (11.8) | 317 (9.8) | 133 (23.8) | <.001 |
| New permanent PM implantation | 600 (18.1) | 465 (16.4) | 135 (27.6) | <.001 |
| Postprocedural AR | ||||
| Moderate | 159 (4.3) | 127 (4.0) | 32 (6.0) | .002 |
| Severe | 19 (0.5) | 12 (0.4) | 7 (1.3) | |
| Postprocedural mean aortic valve gradient, mmHg | 10.8±5.6 | 10.9±5.6 | 10.6±5.7 | .262 |
| Postprocedural peak aortic valve gradient, mmHg | 20.7±10.1 | 20.5±10.5 | 20.9±9.7 | .604 |
| Prosthesis patient mismatch | 312 (9.2) | 283 (9.6) | 29 (6.6) | .043 |
| Length of ICU stay, d | 1 [0-2] | 1 [0-1] | 1 [0-3] | <.001 |
| Length of hospital stay, d | 5 [3-8] | 5 [3-7] | 7 [5-12] | <.001 |
| Clinical outcomes | ||||
| 30-day mortality | 112 (3.0) | 64 (2.0) | 48 (8.5) | <.001 |
| Early safety (at 30 d) | 2799 (73.3) | 2500 (77.0) | 299 (52.1) | <.001 |
| Device success (at 30 d) | 3286 (87.4) | 2865 (89.5) | 421 (75.0) | <.001 |
AKI, acute kidney injury; AR, aortic regurgitation; ICU, intensive/cardiovascular care unit; PM, pacemaker.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
Both early and late thrombocytopenia were associated with worse 30-day clinical outcomes () and increased 30-day mortality compared with patients without thrombocytopenia (adjusted OR, 2.1; 95%CI, 1.0-4.2; P=.041, and 5.4; 95%CI, 2.9-10.0; P <.001), respectively).
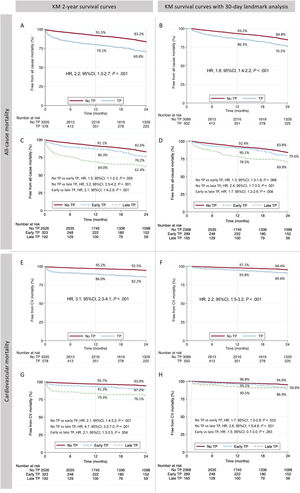
Mid-term outcomesFollow-up was obtained in 3775 (96.5%) patients and the mean follow-up time was 28.5±25.5 months. The 2-year all-cause mortality was 18.9% (30.2% vs 16.8% in patients with and without thrombocytopenia, respectively: hazard ratio [HR], 2.2; 95%IC, 1.8-2.7; P <.001), as shown in figure 3A. After a 30-day landmark analysis this difference remained significant (23.8% vs 15.2%; HR, 1.8; 95%CI, 1.4-2.2; P <.001), as shown in figure 3B. The 2-year cardiovascular mortality was also higher in patients with thrombocytopenia before and after the 30-day landmark analysis (figure 3E,F).
Two-year all-cause mortality and cardiovascular mortality. Kaplan-Meier graph of 2-year all-cause mortality and after 30-day landmark analysis according to thrombocytopenia (A, B) and early and late nadir (C, D). Two-year cardiovascular mortality and after 30-day landmark analysis according to thrombocytopenia and early and late nadir (E-H). CV, cardiovascular; TP, thrombocytopenia.
Among patients with thrombocytopenia, 2-year mortality was significantly higher in those with late nadir compared with early nadir (38.6% vs 23.8%, p<.001, figure 3C and figure 2 – central illustration). This difference remained higher after the 30-day landmark analysis (20.4% vs 30.1%; P=.008, figure 3D). Compared with early nadir, late nadir was associated with increased 2-year cardiovascular death (figure 3G), with the difference mainly observed within the first 30 days (figure 3H).
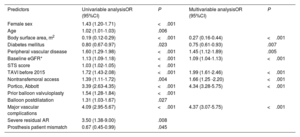
Factors associated with thrombocytopeniaFactors independently associated with thrombocytopenia are presented in table 4. Baseline characteristics that increased the risk of thrombocytopenia were body surface area, absence of diabetes, peripheral vascular disease, and lower estimated glomerular filtration rate (eGFR). Other factors independently associated with thrombocytopenia were procedural aspects, such as TAVI performed in the first half of the study (< 2015), nontransfemoral access, use of the Portico (Abbott, United States) THV, and adverse procedural outcomes, such as major vascular complications.
Independent predictors of thrombocytopenia
| Predictors | Univariable analysisOR (95%CI) | P | Multivariable analysisOR (95%CI) | P |
|---|---|---|---|---|
| Female sex | 1.43 (1.20-1.71) | <.001 | ||
| Age | 1.02 (1.01-1.03) | .006 | ||
| Body surface area, m2 | 0.19 (0.12-0.29) | <.001 | 0.27 (0.16-0.44) | <.001 |
| Diabetes mellitus | 0.80 (0.67-0.97) | .023 | 0.75 (0.61-0.93) | .007 |
| Peripheral vascular disease | 1.60 (1.29-1.98) | <.001 | 1.45 (1.12-1.89) | .005 |
| Baseline eGFR* | 1.13 (1.09-1.18) | <.001 | 1.09 (1.04-1.13) | <.001 |
| STS score | 1.03 (1.02-1.05) | <.001 | ||
| TAVI before 2015 | 1.72 (1.43-2.08) | <.001 | 1.99 (1.61-2.46) | <.001 |
| Nontransfemoral access | 1.39 (1.11-1.72) | .004 | 1.66 (1.25 -2.20) | <.001 |
| Portico, Abbott | 3.39 (2.63-4.35) | <.001 | 4.34 (3.28-5.75) | <.001 |
| Prior balloon valvuloplasty | 1.54 (1.28-1.84) | <.001 | ||
| Balloon postdilatation | 1.31 (1.03-1.67) | .027 | ||
| Major vascular complications | 4.09 (2.95-5.67) | <.001 | 4.37 (3.07-5.75) | <.001 |
| Severe residual AR | 3.50 (1.38-9.00) | .008 | ||
| Prosthesis patient mismatch | 0.67 (0.45-0.99) | .045 |
AR, aortic regurgitation; BSA, body surface area; eGFR, estimated glomerular filtration rate; OR, odds ratio; STS; Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
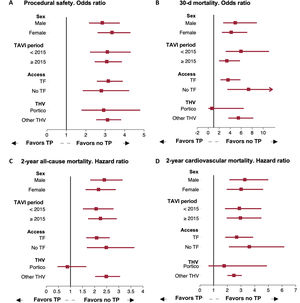
Regarding the type of THV, no significant differences were observed between self-expanding (SEV) and balloon-expandable valves (BEV). However, patients receiving the CoreValve (Medtronic, United States) or Acurate Neo (Boston Scientific, United States) THV had the lowest rates of thrombocytopenia (8.5% and 9.7%, respectively), whereas recipients of the SAPIEN (Edwards System, United States) valve had an intermediate rate (14.2%), and those receiving the Portico valve had the highest occurrence of thrombocytopenia (33.8%) (). Both early and late thrombocytopenia were significantly higher in patients with the Portico valve compared with other THVs (early: 21.3% vs 9.7%; P <.001, and late: 18.3% vs 5.3%, P <.001).
Regarding clinical outcomes, thrombocytopenia did not significantly impact 30-day or 2-year all-cause and cardiovascular mortality among patients receiving the Portico valve (figure 4).
Impact of post-TAVI thrombocytopenia on clinical outcomes by subgroup analysis. Forest plot representing the clinical impact of thrombocytopenia on procedural safety (A), 30-day mortality (B), and 2-year all-cause (C) and cardiovascular mortality (D) in different subgroups. TAVI, transcatheter aortic valve implantation; TF, transfemoral, THV; transcatheter heart valve, TP; thrombocytopenia.
Late thrombocytopenia was associated with increased surgical risk, as assessed by the STS score, and with postprocedural complications including major or life-threatening bleeding, moderate-to-severe aortic regurgitation, and stage 2 or 3 acute kidney injury (AKI) ().
Propensity score matching analysisAfter the propensity score matching analysis, 2 matched groups of 543 patients each, with and without thrombocytopenia were obtained. Baseline and procedural characteristics were balanced between the groups (). The 30-day all-cause mortality and procedural safety remained statistically significantly different between the matched groups with and without thrombocytopenia [39 (7.3%) and 13 (2.5%), P <.001, and 289 (53.6%) and 379 (71.4%), P <.001, respectively, ].
Moreover, 2-year all-cause and cardiovascular mortality were significantly higher in the propensity score matched group with thrombocytopenia compared with those without thrombocytopenia (29.2% vs 16.7%; HR, 2.0; 95%CI, 1.5 - 2.7; P <.001, and 16.5% vs 7.2%, HR, 2.7; 95%CI, 1.7-4.4; P <.001, respectively, ).
Subgroup analysisThe clinical impact of thrombocytopenia on procedural safety, 30-day mortality, and 2-year all-cause and cardiovascular mortality was consistently observed in all subgroups according to sex, year of TAVI, and access route. However, in the Portico THV subgroup no significant impact of thrombocytopenia on short or mid-term mortality was observed (figure 4).
DISCUSSIONIn this large multicenter registry including 3913 patients treated with TAVI for aortic valve stenosis, we aimed to assess the risk factors and clinical impact of thrombocytopenia after the procedure. Major findings were a DPC of approximately 35% on day 3 post-TAVI, with an incidence of thrombocytopenia (DPC ≥ 50%) of around 15% (early ∼60% and late ∼40%). A subsequent increase in platelet count occurred in 63% of all patients, and in 79% of those with a hospital stay of ≥ 4 days, suggesting a transient phenomenon in most patients.
Thrombocytopenia was associated with increased periprocedural complications, 30-day mortality and 2-year mortality, both before and after propensity score matching analysis. Both early and late thrombocytopenia were independently associated with 30-day mortality. However, only late thrombocytopenia was associated with mid-term survival in a landmark analysis after 30 days. Independent factors associated with thrombocytopenia included baseline characteristics (body surface area, absence of diabetes mellitus, lower eGFR, and peripheral vascular disease) as well as procedural factors (procedures performed before 2015, no transfemoral access, vascular complications, and THV type).
The incidence of thrombocytopenia after TAVI has been previously reported range from 25% to 65%.4,5,9–11 However, the definition of thrombocytopenia varies considerably among studies, with criteria including platelet nadir <150 *109/L, or <100 *109/L and DPC thresholds of ≥50%, ≥46% or ≥30%. A meta-analysis of 9 studies with 2278 patients reported an incidence of major thrombocytopenia of 44.9%,12 using several definitions (DPC ≥30%, DPC ≥50%, nadir platelet count <100*109/L, and second or third tercile DPC). DPC has been suggested to be a stronger predictor for short-term survival and major adverse cardiovascular events compared with platelet count nadir.5,10 Therefore, our study used DPC ≥ 50% as the definition of thrombocytopenia, and confirmed that it is not only a frequent complication but is also clearly associated with worse clinical outcomes.
The incidence of thrombocytopenia in our cohort was similar to that observed in aortic valve surgery,13 even through TAVI procedures do not involve cardiopulmonary bypass and have milder degrees of hemodilution or bleeding with less platelet consumption. Potential mechanisms that may lead to the high incidence of post-TAVI thrombocytopenia include damage to the endothelium, altered shear stress, exposure of the stent surface with possible activation of the immune system and platelet consumption, and the reduced ability of elderly patients to regenerate platelets. Heparin-induced thrombocytopenia could also contribute to significant DPC, although it has been reported in less than 1% of TAVI patients.14 In addition, more aggressive and complex cases (eg, transapical approach, higher use of contrast agents, and vascular complications) may further increase platelet activation, potentially explaining the association between these factors and the thrombocytopenia observed in our cohort.
Previous studies have reported a higher incidence of thrombocytopenia in patients with BEV compared with SEV.11,15 However, these finding were not confirmed in our large multicenter study. Interestingly, we found differences in the incidence of thrombocytopenia according to different types of THV. Agents used for valve preservation or anti-calcification systems have been suggested in the etiopathogenesis of thrombocytopenia,16 which might explain these differences between THV types. Interestingly, this finding did not result in worse in-hospital outcomes compared with other devices.
According to previous studies, the association between thrombocytopenia and clinical outcomes is controversial. Two studies with BEV (> 30% transapical access), did not observe a significant impact of thrombocytopenia on short-term mortality.9,17 In contrast, 2 other small single-center studies reported an increased 30-day and 1-year mortality in patients with severe thrombocytopenia after TAVI.4,11 The aforementioned meta-analysis also found a higher risk of 30-day mortality in patients with thrombocytopenia.12
Our cohort included a larger and more contemporary TAVI population, with a higher use of transfemoral access and SEV, and also demonstrated a higher risk of 30-day and 2-year all-cause mortality in patients with thrombocytopenia. This association persisted even after propensity score matching analysis, suggesting that acquired thrombocytopenia is an independent predictor of adverse clinical outcomes. The higher risk of periprocedural hemorrhagic and ischemic complications, as well as AKI, is also of concern in patients with postprocedural thrombocytopenia.
While bleeding complications seem to be strongly associated with thrombocytopenia,12,18 establishing a definitive cause-and-effect relationship is difficult. An association between thrombocytopenia and AKI has been suggested in patients undergoing cardiac surgery, where microaggregates of activated platelets, endothelial cells, and white blood cells may clog glomerular capillaries, subsequently inducing AKI.19 This mechanism might be involved in other ischemic injuries, such as stroke.
The dynamic platelet count over time is a known predictor of outcomes in intensive care patients. In our cohort, patients with a late nadir had a higher risk of 30-day and 2-year all-cause and cardiovascular mortality. Early nadir with prompt platelet recovery likely represents the “expected” platelet count behavior, primarily due to hemodilution and consumption in the context of an invasive procedure.
In contrast, late thrombocytopenia may be secondary to a destructive etiology, such as immune thrombocytopenia, late infection, or another late complication. It may also reflect a failure of platelet recovery in elderly patients with additional comorbidities and/or increased frailty, explaining its greater prognostic impact. Further studies are needed to explore the underlying causes and potential targeted therapies for this complication, to improve outcomes in patients with late-acquired thrombocytopenia after TAVI.
LimitationsWe acknowledge several limitations of this study, which are partly due to its retrospective design. First, no additional complementary testing was conducted to clarify the etiology of thrombocytopenia, particularly in cases of late thrombocytopenia or heparin-induced thrombocytopenia. Second, despite multivariable and propensity score matching analyses, confounding factors related to the long inclusion period, procedural approaches, and peri-procedural complications cannot be entirely ruled out. Third, in-hospital infections and sepsis were not prospectively collected and, therefore, could not be analyzed as potential contributors to acquired thrombocytopenia. Fourth, shorter hospital stays for patients undergoing minimally invasive TAVI may have led to bias due to the absence of platelet count measurements postdischarge. Finally, the multicenter design and extended inclusion period introduced variability in technical approaches and pharmacological therapies, including antithrombotic regimens. However, this also enhances the external validity of our results, as participating centers adhered to the guidelines of the American and European Cardiology Societies.
CONCLUSIONSAcquired thrombocytopenia was relatively common following TAVI and was linked to an increased risk of periprocedural complications as well as short- and mid-term mortality. Among patients with thrombocytopenia, those with a late nadir experienced a higher mortality risk than those with an early nadir. The development of thrombocytopenia was associated with baseline comorbidities, procedural factors such as TAVI access, type of THV, and procedural complications. Observed differences in thrombocytopenia rates among various THVs warrant further investigation in future studies.
FUNDINGThere was no dedicated funding for this study.
ETHICAL CONSIDERATIONSAll patients provided informed consent before the procedure, and the study was conducted in accordance with the institutional review board at each participating center. The research adhered strictly to the SAGER (Sex and Gender Equity in Research) guidelines to address potential sex and gender biases. This study was carried out without direct patient involvement; patients were not invited to comment on the study design, nor were they consulted in developing patient-relevant outcomes or interpreting the results.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEThis research did not use artificial intelligence technologies or tools at any stage. All data collection, analysis, and interpretation were conducted manually by the research team, ensuring the integrity and accuracy of the findings.
AUTHORS’ CONTRIBUTIONSG. Tirado-Conte and L. Nombela-Franco: substantial contributions to the conception or design of the work; acquisition, analysis, or interpretation of data; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rest of the authors: Drafting the work or revising it critically for important intellectual content and final approval of the version to be published.
CONFLICTS OF INTERESTG. Tirado-Conte has received a research-training contract (Rio Hortega, CM21/00091) from the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III).
K. Arslani has received a research grant from the Swiss Academy of Medical Sciences, the Gottfried and Julia Bangerter-Rhyner Foundation, and the Swiss National Science Foundation (P500PM_202963), outside of the submitted work.
L. Nombela-Franco is a proctor for Abbott Vascular and Edwards Lifesciences.
- -
Acquired thrombocytopenia is a common but poorly-understood complication following TAVI, with its impact on short- and mid-term survival remaining unclear.
- -
The temporal pattern of platelet count after TAVI may be associated with different clinical outcomes.
- -
Acquired thrombocytopenia occurred in nearly 15% of patients after TAVI. Platelet nadir was observed on day 3 postprocedure, with recovery seen in up to 78% of patients after ≥ 4 days.
- -
Thrombocytopenia was associated with a decrease in procedural safety and higher risk of both 30-day and 2-year mortality.
- -
Late compared with early thrombocytopenia was associated with reduced short- and mid-term survival.
- -
Independent predictors of thrombocytopenia were body surface area, renal function, nontransfemoral access, valve type, and vascular complications.
- -
Thrombocytopenia should be regarded as a predictor of worse clinical outcomes after TAVI. Further research is needed to explore its etiology, prevention, and management.