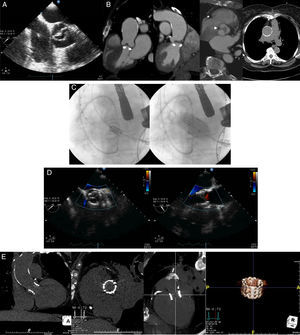
A 71-year-old woman diagnosed with symptomatic aortic stenosis (exertional dyspnea, New York Heart Association class III) and hostile chest was referred to our hospital for transcatheter aortic valve replacement (TAVR). She had undergone off-pump coronary bypass artery grafting several years previously, complicated with severe mediastinitis and sternal dehiscence requiring surgical intervention and chest reopening. She also had systemic arterial hypertension, type 2 diabetes, peripheral vascular disease, and chronic renal failure leading to an estimated risk of perioperative mortality of 21% and 6% as assessed by Logistic EuroSCORE and Society of Thoracic Surgeons score, respectively. The echocardiographic examination revealed a noncalcific bicuspid aortic valve with severe stenosis (mean gradient, 41mmHg; valve area as assessed by the continuity equation, 0.54 cm2) (Figure A), and a left ventricular ejection fraction of 60%. A multidetector computed tomography confirmed severe thickening of the leaflets and the absence of calcium on the aortic valve (Figure B), severe calcification of the ascending aorta without significant dilatation (Figure B) and severe peripheral vascular disease with concentric calcification of both iliofemoral arteries and a minimal luminal diameter of 5.5 and 4.7mm in the right and left side, respectively. The patient was deemed unsuitable for standard aortic valve surgery by the heart team, and TAVR using the transapical approach was proposed. The case was approved by the Special Access Program for compassionate clinical use of Health Canada, and the patient provided signed informed consent for the procedure. According to the assessment of the dimensions of the aortic annulus by multidetector computed tomography (21 x 27mm; area, 4.7 cm2), a 26-mm balloon-expandable Edwards SAPIEN-XT valve (Edwards Lifesciences, Irvine, California, United Sates) was selected and, following balloon valvuloplasty with a 20-mm balloon, the transcatheter valve was successfully implanted (Figure C). The echocardiographic examination post-TAVR showed the absence of residual aortic regurgitation (Figure D) and a valve area of 1.31 cm2. At 1-month follow-up, a multidetector computed tomography showed both the adequate positioning and uniform expansion of the bioprosthesis (Figure E). Valve hemodynamics remained unchanged and the patient was asymptomatic at the 9-month follow-up.
A: transesophageal echocardiography mid-esophageal aortic short axis view showing a noncalcific bicuspid aortic valve. B: computed tomography images assessing the aortic valve and ascending aorta: coronal oblique reconstructions showing the absence of calcium on the aortic valve and the severe thickness of the cusps (arrows) and double oblique transverse view showing the presence of a thick raphe (arrowhead) and the asymmetric opening of the aortic valve; axial view displaying a severe calcified ascending aorta. C: fluoroscopic images showing the positioning, using a pigtail catheter placed on the aortic valve as reference, and deployment of a 26-mm balloon-expandable valve by transapical approach. D: postprocedural transesophageal echocardiography mid-esophageal long-axis and short-axis views showing the absence of residual aortic regurgitation. E: 30 days post implantation computed tomography coronal and sagital views, coronal oblique reconstruction views and volume-rendered image showing the complete expansion and correct positioning of the stent valve.
Biscupid aortic valve disease (BAVD) is the most common congenital heart defect and the first cause of aortic stenosis requiring aortic valve replacement. Although most cases occur in calcified valves, severe aortic stenosis in BAVD may occur in thick and fibrous valves lacking calcium, which is more frequent in younger patients.
Both the presence of a BAVD and the absence of calcium on the aortic valve are contraindications for TAVR1 due to the potential risk of valve dislodgment. Valve calcification is considered to be a necessary condition for the anchoring of the valve stent frame, which might be even more relevant with the use of balloon-expandable valves. However, studies in animal models have shown that an accurate sizing of the valve with a higher degree of prosthesis oversizing may prevent device migration in valves without calcium.2
The use of self-expandable bioprostheses in noncalcified aortic valves allows a high oversizing with a minimal risk of annulus rupture. Nonetheless, the higher radial force of the balloon-expandable valves3 may allow an appropriate anchoring of the bioprosthesis in noncalcified valves with less oversizing.2 In this case, a relative oversizing of 13% (within the recommended range of 10%-15%) was enough to prevent bioprosthesis embolization,4 but future studies will have to determine the degree of oversizing which should be used in these cases. Also, the eccentricity of the aortic annulus and the severe thickness of the leaflets in this patient might have contributed to resistance to migration forces. Moreover, the use of self-expandable bioprostheses in patients with BAVD has been associated with a greater eccentricity,5 which in turn might lead to a higher peak stress on the leaflets and a higher risk of central and paravalvular leak.6
Several series have reported the feasibility of TAVR in patients with calcified BAVD.5 However, this report shows for the first time that TAVR with the use of balloon-expandable valves can be successfully performed for the treatment of noncalcified BAVD, suggesting that TAVR might be a therapeutic alternative in selected patients with congenital aortic valve disease without valve calcification. Further studies are warranted.
CONFLICTS OF INTERESTDr Josep Rodés-Cabau is consultor for Edwards Lifesciences and St-Jude Medical. Dr. Eric Dumont is consultor for Edwards Lifesciences