To evaluate whether a genetic risk score (GRS) improves prediction of recurrent events in young nondiabetic patients presenting with an acute myocardial infarction (AMI) and identifies a more aggressive form of atherosclerosis.
MethodsWe conducted a prospective study with consecutive nondiabetic patients aged <55 years presenting with AMI. We performed a genetic test, cardiac computed tomography, and analyzed several biomarkers. We studied the association of a GRS composed of 11 genetic variants and a primary composite endpoint (cardiovascular mortality, a recurrent event, and cardiac hospitalization).
ResultsA total of 81 patients were studied and followed up for a median of 4.1 years. There were 24 recurrent cardiovascular events. Compared with the general population, study participants had a higher prevalence of 9 out of 11 risk alleles. The GRS was significantly associated with recurrent cardiovascular events, especially when baseline low-density lipoprotein cholesterol (LDL-C) levels were elevated. Compared with the low-risk GRS tertile, the multivariate-adjusted HR for recurrences was 10.2 (95%CI, 1.1-100.3; P=.04) for the intermediate-risk group and was 20.7 (2.4-181.0; P=.006) for the high-risk group when LDL-C was≥2.8mmol/L (≥ 110mg/dL). Inclusion of the GRS improved the C-statistic (ΔC-statistic=0.086), cNRI (continuous net reclassification improvement) (30%), and the IDI (integrated discrimination improvement) index (0.05). Cardiac computed tomography frequently detected coronary calcified atherosclerosis but had limited value for prediction of recurrences. No association was observed between metalloproteinases, GRS and recurrences.
ConclusionsA multilocus GRS may identify individuals at increased risk of long-term recurrences among young nondiabetic patients with AMI and improve clinical risk stratification models, particularly among patients with high baseline LDL-C levels.
Keywords
Coronary artery disease (CAD) remains the first cause of mortality worldwide.1 CAD has a complex etiology involving environmental risk factors and genetic susceptibility. Genetic predisposition has been stated to account for 40% to 50% of variability in the development of CAD.2 Young patients (<55 years) with an acute myocardial infarction (AMI) usually lack most of the clinical risk factors and almost invariantly have low cardiovascular risk based on clinical estimators until the moment of the event. Genome-wide association studies (GWAS) have consistently identified more than 57 loci in the general population that predispose to CAD/AMI either through cardiovascular risk factors or independently.3 The importance of genetically derived etiopathogenic mechanisms of CAD is enhanced among patients with early clinical events. Nevertheless, clinical guidelines do not currently recommend the use of genetic testing to assess cardiovascular risk in primary or secondary prevention.4,5
CAD is not uncommon in the young population, and patients <45 years represent approximately 10% of AMI admissions,6 with a significantly high lifelong chance of recurrent cardiovascular events despite therapy.7,8
Classical cardiovascular risk factors are of great importance as therapeutic targets after an AMI and have also been used to design risk prediction scores for further coronary events (ie, Global Registry of Acute Coronary Events [GRACE]). However, their predictive ability is limited and they do not account for genetically derived risk.9
The association of genetic scores with incident CAD has been prospectively studied in the general population, with overall positive results. Advances in genetic testing have made these studies affordable, although cost-effectiveness remains an issue.10,11
Currently, there is an unmet need to identify patients with established cardiovascular disease who are at higher risk of recurrent cardiovascular events and who could benefit from more aggressive secondary prevention. Young patients with cardiovascular disease emerge as one of the main focuses of attention, since they have an unfavourable long-term prognosis with a high probability of further recurrences.12 In this context, genetic risk scores (GRS) composed of single-nucleotide polymorphisms (SNPs) might be clinically useful to assess lifelong residual risk.
We sought to determine whether an SNP-based GRS could predict long-term recurrent cardiovascular events after an AMI in a cohort of young nondiabetic patients with a high likelihood of genetic susceptibility and could therefore improve risk stratification. We also aimed to determine whether a poor GRS was associated with a more aggressive form of CAD through a comprehensive study of plaque morphology and coronary calcium by computed tomography (CT), invasive angiography, and biohumoral extracellular matrix metabolism parameters.
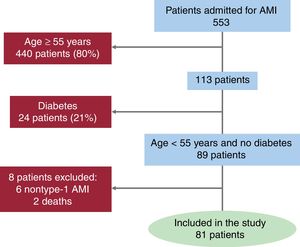
METHODSStudy designWe conducted a prospective study with consecutive inclusion of nondiabetic patients aged <55 years hospitalized for type 1 AMI in a tertiary hospital. Inclusion criteria were: a) age between 18 and 55 years; b) type 1 AMI presenting as non–ST-segment elevation or ST-segment elevation acute coronary syndrome (ACS) and, c) invasive coronary angiography performed. Exclusion criteria were: a) age>55 years, and b) a clinical history of diabetes or fulfilment of established diagnostic criteria according to the European Society of Cardiology/European Association for the Study of Diabetes guidelines. We obtained comprehensive clinical, demographic, echocardiographic, and invasive angiographic data. Blood samples for genetic testing and metalloproteinase analysis were extracted at admission and were stored at the local biobank. A cardiac CT for calcium score analysis and noninvasive coronary angiography was performed at baseline unless contraindicated. The study protocol was approved by the ethics committee (reference 175/13) and complied with the Declaration of Helsinki. All participants provided written informed consent.
OutcomeThe primary outcome, defined as a recurrent cardiovascular event, was a composite of cardiovascular mortality, recurrent ACS, and cardiac hospitalization. Recurrent ACS included both AMI and unstable angina. Ambulatory staged procedures for revascularization of nonculprit lesions were not considered as recurrences. All outcomes were reviewed by 2 cardiologists. Follow-up was performed on site every 6 months. Covariate definitions were standardized for analysis.
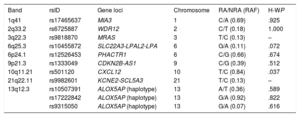
GenotypingDNA extraction was performed using standard techniques. Genotyping was conducted at the Gen inCode Laboratory (Barcelona, Spain), using designed Affymetrix Axiom arrays.3 The genome-wide arrays gave high-quality genotypes, with high genotype call rates (100%) and SNP reproducibility (100%). Genotype imputation was done on a per-array basis using IMPUTE2 v2.2.2 and using 1000 genomes as the reference panel. Genetic variants (table 1) were determined using a commercial platform named Cardio inCode Score (Gen inCode, Spain), which includes 11 genetic variants associated with CAD but not with classic risk factors in accordance with the data available at genome-wide association studies catalogue reviewed in August 2010 and which were described in GWAS (rs10455872 in LPA, rs12526453 in PHACTR1, rs1333049 in CDKN2A/B, rs17465637 in MIA3, rs501120 in CXCL12, rs6725887 in WDR12, rs9818870 in MRAS and rs9982601 in SLC5A3/KCNE2) including also a haplotype in ALOX5AP gene (Hap B, composed by: rs10507391-A, rs93155050-A and rs17222842-G), which has been reported to be associated with CAD in different populations.13–15 This haplotype was not found in GWAS because individual genetic variants are usually analyzed without taking haplotypes into consideration. The array yielded high genotype call rates (100%). The exact test was used to compute Hardy-Weinberg equilibrium in each individual variant considering a P value <0.0511 for statistical significance in multiple testing.16
Genetic variants analyzed
| Band | rsID | Gene loci | Chromosome | RA/NRA (RAF) | H-WP |
|---|---|---|---|---|---|
| 1q41 | rs17465637 | MIA3 | 1 | C/A (0.69) | .925 |
| 2q33.2 | rs6725887 | WDR12 | 2 | C/T (0.18) | 1.000 |
| 3q22.3 | rs9818870 | MRAS | 3 | T/C (0.13) | – |
| 6q25.3 | rs10455872 | SLC22A3-LPAL2-LPA | 6 | G/A (0.11) | .072 |
| 6p24.1 | rs12526453 | PHACTR1 | 6 | C/G (0.66) | .674 |
| 9p21.3 | rs1333049 | CDKN2B-AS1 | 9 | C/G (0.39) | .512 |
| 10q11.21 | rs501120 | CXCL12 | 10 | T/C (0.84) | .037 |
| 21q22.11 | rs9982601 | KCNE2-SCL5A3 | 21 | T/C (0.13) | – |
| 13q12.3 | rs10507391 | ALOX5AP (haplotype) | 13 | A/T (0.36) | .589 |
| rs17222842 | ALOX5AP (haplotype) | 13 | G/A (0.92) | .822 | |
| rs9315050 | ALOX5AP (haplotype) | 13 | G/A (0.07) | .616 |
H-W, Hardy-Weinberg equilibrium; NRA, nonrisk allele; RA, risk allele; RAF: risk allele frequency.
P <.0045 for statistical significance.
A GRS was computed using the following formula GRS =∑i=1nSNPi where the sum of the number of risk alleles described in table 1 (with values 0, 1, or 2) across the genetic variants (n) was included. Patients were divided into terciles based on their GRS for analysis.
Cardiac computed tomographyA cardiac CT scan was performed after myocardial revascularization and before hospital discharge in all participants unless contraindicated. Coronary artery calcium (CAC) score as well as noninvasive coronary angiography were performed using a 128-slice multidetector CT. The scans were read independently by 2 investigators. The methodology for CAC acquisition and interpretation of the CT scans was performed according to a validated standardized protocol.17 The amount of calcium was quantified with the Agatston scoring method, excluding stents from the analysis.
Extracellular matrix metabolism biomarkersVenous blood samples were collected during the first 24 hours after admission. Within 30 minutes after extraction, centrifugation was performed at 100 g for 15 minutes. Serum was removed and stored at −80°C at the local biobank. Metalloproteinase (MMP) 1, 2, 7, 9 and 10, and tissue inhibitor of matrix metalloproteinase 1 (TIMP-1) assays were performed at the Atherosclerosis Research Laboratory, CIMA-University of Navarre (Pamplona, Spain). MMP 1, 2, 7, 9 and 10 were analyzed in serum with a bead-based multiplex assay using the Luminex technology, from Merck (MILLIPLEX MAP, Darmstadt, Germany). TIMP-1 levels were assayed by enzyme-linked immunosorbent assay (Quantikine; R&D Systems, Minneapolis, Minnesota, United States). Interassay and intra-assay coefficients of variation were <6%.
Statistical analysisFor statistical analysis, the GRS was divided into 3 terciles and Kaplan-Meier survival curves were plotted for the primary outcome. The low-risk GRS was used as the reference group. Several Cox regression models were constructed: a) a univariate model with all the variables of interest collected; b) a multivariate clinical model where the already established GRACE risk score and variables that were statistically associated with the composite endpoint in the univariate analysis were tested; the backward elimination method was used for selection of covariables, and c) the previous multivariate model with the addition of GRS terciles. Interaction was studied. The percent risk for the GRACE risk score was calculated using the nomogram for the 3-year outcomes of death or AMI. Goodness of fit was evaluated using the likelihood ratio test and the C-statistic. Multivariate models with and without GRS were compared by the integrated discrimination improvement index (IDI) method, which compares the average difference in correctly predicting the risk for patients who have a recurrent cardiovascular event with those who do not, and the continuous net reclassification improvement (cNRI), detailed in .18 Missing values (< 3%) were sampled using a multiple imputation technique based on the Markov method. This was a hypothesis-generating and exploratory study; therefore, no formal sample size calculation was performed in advance. The statistical analyses were performed using SPSS software version 22.0 (SPSS Inc, Chicago, Illinois, United States) and R version 3.3.2.
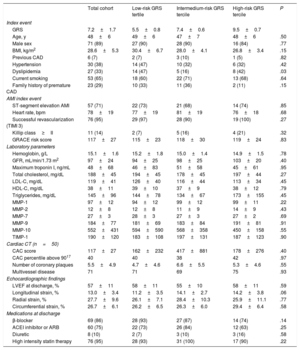
RESULTSPatient profileFrom August 2013 through December 2014, a total of 81 patients (89% male) aged 48±6 years were enrolled into the study after screening (figure 1). Cardiovascular risk factors and clinical features are shown in table 2 and . The most common risk factor was smoking (65%) followed by hypertension and dyslipidemia in 38% and 33%, respectively. One in every 4 patients was in the obesity range, and 29% reported a family history of premature CAD (AMI in first-degree male relatives aged <55 years or female relatives aged <65 years). All patients were of European ancestry and none of them had familial hypercholesterolemia according to the Dutch Lipid Clinic Network criteria ().
Baseline patient characteristics according to their genetic risk score tercile (n=81)
| Total cohort | Low-risk GRS tertile | Intermedium-risk GRS tercile | High-risk GRS tercile | P | |
|---|---|---|---|---|---|
| Index event | |||||
| GRS | 7.2±1.7 | 5.5±0.8 | 7.4±0.6 | 9.5±0.7 | |
| Age, y | 48±6 | 49±6 | 47±7 | 48±6 | .50 |
| Male sex | 71 (89) | 27 (90) | 28 (90) | 16 (84) | .77 |
| BMI, kg/m2 | 28.6±5.3 | 30.4±6.7 | 28.0±4.1 | 26.8±3.4 | .15 |
| Previous CAD | 6 (7) | 2 (7) | 3 (10) | 1 (5) | .82 |
| Hypertension | 30 (38) | 14 (47) | 10 (32) | 6 (32) | .42 |
| Dyslipidemia | 27 (33) | 14 (47) | 5 (16) | 8 (42) | .03 |
| Current smoking | 53 (65) | 18 (60) | 22 (71) | 13 (68) | .64 |
| Family history of premature CAD | 23 (29) | 10 (33) | 11 (36) | 2 (11) | .15 |
| AMI index event | |||||
| ST-segment elevation AMI | 57 (71) | 22 (73) | 21 (68) | 14 (74) | .85 |
| Heart rate, bpm | 78±19 | 77±19 | 81±19 | 76±18 | .68 |
| Successful revascularization (TIMI 3) | 76 (95) | 29 (97) | 28 (90) | 19 (100) | .27 |
| Killip class≥II | 11 (14) | 2 (7) | 5 (16) | 4 (21) | .32 |
| GRACE risk score | 117±27 | 115±23 | 118±30 | 119±24 | .83 |
| Laboratory parameters | |||||
| Hemoglobin, g/L | 15.1±1.6 | 15.2±1.8 | 15.0±1.4 | 14.9±1.5 | .78 |
| GFR, mL/min/1.73 m2 | 97±24 | 94±25 | 98±25 | 103±20 | .40 |
| Maximum troponin I, ng/mL | 48±68 | 46±83 | 51±58 | 45±61 | .95 |
| Total cholesterol, mg/dL | 188±45 | 194±45 | 178±45 | 197±44 | .27 |
| LDL-C, mg/dL | 119±41 | 126±40 | 116±44 | 113±34 | .45 |
| HDL-C, mg/dL | 38±11 | 39±10 | 37±9 | 38±12 | .79 |
| Triglycerides, mg/dL | 145±96 | 144±78 | 134±67 | 173±155 | .45 |
| MMP-1 | 97±12 | 94±12 | 99±12 | 99±11 | .22 |
| MMP-2 | 12±8 | 12±8 | 11±9 | 14±9 | .43 |
| MMP-7 | 27±3 | 28±3 | 27±3 | 27±2 | .69 |
| MMP-9 | 184±77 | 181±69 | 183±84 | 191±81 | .91 |
| MMP-10 | 552±431 | 594±590 | 568±358 | 450±158 | .55 |
| TIMP-1 | 190±120 | 183±108 | 197±131 | 187±123 | .90 |
| Cardiac CT (n=50) | |||||
| CAC score | 117±27 | 162±232 | 417±881 | 178±276 | .40 |
| CAC percentile above 9017 | 40 | 40 | 38 | 42 | .97 |
| Number of coronary plaques | 5.5±4.9 | 4.7±4.6 | 6.6±5.5 | 5.3±4.6 | .55 |
| Multivessel disease | 71 | 71 | 69 | 75 | .93 |
| Echocardiographic findings | |||||
| LVEF at discharge, % | 57±11 | 58±11 | 55±10 | 58±11 | .59 |
| Longitudinal strain, % | 13.0±3.4 | 11.2±3.5 | 14.1±2.7 | 14.2±3.8 | .06 |
| Radial strain, % | 27.7±9.6 | 26.1±7.1 | 28.4±10.3 | 25.9±11.1 | .77 |
| Circumferential strain, % | 26.7±6.1 | 26.2±6.5 | 26.3±6.0 | 29.4±6.4 | .58 |
| Medications at discharge | |||||
| β-blocker | 69 (86) | 28 (93) | 27 (87) | 14 (74) | .14 |
| ACEI inhibitor or ARB | 60 (75) | 22 (73) | 26 (84) | 12 (63) | .25 |
| Diuretic | 8 (10) | 2 (7) | 3 (10) | 3 (16) | .58 |
| High intensity statin therapy | 76 (95) | 28 (93) | 31 (100) | 17 (90) | .22 |
ACEI, angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin-II receptor blocker; BMI, body mass index; CAC, coronary artery calcium; CAD, coronary artery disease; CT, computed tomography; GFR, glomerular filtration rate; GRS, genetic risk score; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MMP, metalloproteinase; TIMI, Thrombolysis in Myocardial Infarction; TIMP-1, tissue inhibitor of matrix metalloproteinase 1.
Values are expressed as mean±standard deviation for continuous variables and No. (%) for categorical variables.
ST-segment elevation AMI was the most frequent presentation accounting for 72% of cases. The median [IQR] hospitalization time was 2.5 [2-4] days. A total of 32% had severe nonculprit stenosis determined by invasive angiography, and 4% had 3-vessel disease. Discharge treatment met current guideline recommendations.
After a median [IQR] follow-up of 4.1 [3.5-4.4] years, there were 24 occurrences (30%) of the primary composite endpoint. Morbidity and mortality was almost exclusively cardiac. Three-year follow-up was completed in all participants, and among patients with recurrent cardiovascular events, the mean time to the primary endpoint was 1.1±0.8 years.
In the univariate analysis, a previous history of CAD (hazard ratio [HR], 3.4, 95% confidence interval [95%CI], 1.2-9.9; P=.02), dyslipidemia (HR, 5.0; 95%CI, 1.8-14.2; P=.001) and previous cocaine abuse (HR, 5.1, 95%CI, 1.7-15.1; P=.001) were significantly associated with the composite endpoint. Clinical features at admission that were associated with recurrent cardiovascular events were Killip class (HR, 2.3 per point increase; 95%CI, 1.4-3.7; P <.001), suboptimal revascularization defined as final TIMI (Thrombolysis in Myocardial Infarction) flow <3 (HR, 8.9; 95%CI, 2.9-26.9; P=.01), a low hemoglobin count (HR, 1.4 per point decrease; 95%CI, 1.2-1.7; P <.001), and need of diuretic drugs during the index event (HR, 4.2; 95%CI, 1.5-11.3; P <.002).
A multivariate clinical model was constructed, testing all significant variables in the univariate analysis. Only GRACE risk score and low-density lipoprotein cholesterol (LDL-C) levels remained in the final model, which showed an area under the curve of 0.78 for the prediction of the primary endpoint at 3 years in the ROC (receiver operating characteristic) curve.
GeneticsThe prevalence of each SNP that composed the GRS was evaluated in our population in comparison with a cohort of more than 15 400 exome sequences from unrelated individuals of European non-Finnish origin included in the Genome Aggregation Database (gnomAD). Our population had a consistently higher prevalence in 9 out of the 11 risk alleles analyzed ().
When the 8 SNPs and the 3 haplotype variants were individually examined, none of them were found to have statistical association with recurrent cardiovascular events after application of the Bonferroni correction for multiple hypotheses ().
The mean GRS in our population was 7.2±1.7. When divided into terciles based on GRS, the characteristics of the index event were not significantly different (table 2). As expected by design (ie, selection of SNP components in the Cardio inCode platform), GRS was not associated with the classic risk factors or a family history of premature CAD. The validated GRACE risk prediction score determined at inclusion did not differ depending on the genetic risk tercile.
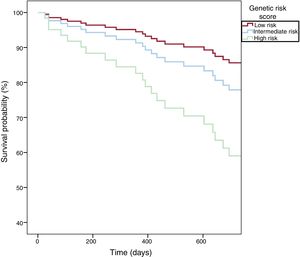
We found a significant association between the GRS and recurrent cardiovascular events in the multivariate Cox model (which included GRACE and LDL-C) (table 3 results of the supplementary data, and ). Kaplan-Meier curves showed a clear pattern toward higher recurrent cardiovascular events for intermediate and high-risk GRS patients (figure 2). LDL-C levels determined at baseline showed a strong interaction effect among patients with high-risk GRS and consequently, in this group, elevated levels of LDL-C increased their HR of recurrent cardiovascular events (). Among patients with baseline LDL-C≥110mg/dL (≥ 2.8 mmol/L), the multivariable-adjusted HR for recurrent cardiovascular events was 10.2 (95%CI, 1.1-100.3; P=.04) for the intermediate genetic risk category and was 20.7 (2.4-181.0; P=.006) for the high-risk GRS compared with the low-risk GRS category. In contrast, the genetic risk category did not contribute to the prediction of recurrences when LDL-C was <110 mg/dL (2.8 mmol/L). There were no significant differences in absolute or relative LDL-C reduction at 6 months based on GRS tertile ().
Cox regression analysis between GRS terciles and recurrent risk of events
| Univariate analysis | Multivariate analysis* | ||||
|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P* | ||
| GRS | |||||
| Low GRS | 1 | 1 | |||
| Intermediate GRS | 2.0 (0.7-5.8) | .21 | LDL-C≤110 mg/dL (≤ 2.8 mmol/L) | 1.0 (0.3-4.0) | |
| >110 mg/dL (≥ 2.8 mmol/L) | 10.2 (1.1-100.3) | .04 | |||
| High GRS | 3.0 (1.0-9.2) | .05 | LDL-C≤110 mg/dL (≤ 2.8 mmol/L) | 0.3 (0.1-1.9) | |
| > 110 mg/dL (≥ 2.8 mmol/L) | 20.7 (2.4-181.0) | .006 | |||
95%CI, 95%, confidence interval; GRACE, Global Registry of Acute Coronary Events; GRS, genetic risk score; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol.
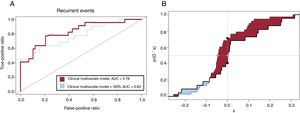
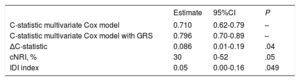
The addition of the GRS to the final multivariate clinical Cox model (which included GRACE risk score and LDL-C) showed an improvement in the area under the curve (0.83 vs 0.78) (figure 3A). The C-statistic for the clinical multivariate Cox model was 0.710, while the addition of the GRS increased it to 0.796 (Δc- statistic=0.086; 95%CI, 0.006-0.187). The cNRI was 30% at 3 years (95%CI, 0-52%, P=.05) while the IDI showed a substantial incremental predictive ability (0.05) when the GRS was added to the clinical multivariate model (figure 3B and table 4).
A: receiver operating characteristic curves for the multivariate Cox model (including GRACE risk score and LDL-C) in red and for the multivariate model with the addition of the GRS in blue. B: empirical distribution function of ^D for T0 ≤ t0 (thick solid line) and T0> t0 (thin solid line) for assessing IDI and cNRI, as described by Uno et al18. T0 denotes event time, while t0 reflects a particular time point. Patients who have an event by t0 can be defined as cases (ie, T0 ≤ t0) and those who do not as controls (ie, T0> t0). ^D denotes the change between the 2 models and visually assesses improvement in risk prediction systems (see ). AUC, area under the curve; cNRI, continuous net reclassification improvement; GRACE, Global Registry of Acute Coronary Events; GRS, genetic risk score; IDI, integrated discrimination improvement; LDL-C, low-density lipoprotein cholesterol.
Evaluation of the incremental value of GRS to the multivariate model
| Estimate | 95%CI | P | |
|---|---|---|---|
| C-statistic multivariate Cox model | 0.710 | 0.62-0.79 | – |
| C-statistic multivariate Cox model with GRS | 0.796 | 0.70-0.89 | – |
| ΔC-statistic | 0.086 | 0.01-0.19 | .04 |
| cNRI, % | 30 | 0-52 | .05 |
| IDI index | 0.05 | 0.00-0.16 | .049 |
95%CI, 95% confidence interval; cNRI, continuous net reclassification index; IDI, integrated discrimination improvement; GRS, genetic risk score.
The Table shows the c-statistic and the IDI index and the cNRI for comparing the 2 multivariate Cox regression models with vs without the GRS with censored survival data.
Cardiac CT was performed in 50 patients (62%). The CAC score was high (117±27), with only 30% of patients free of additional coronary calcification. More than two thirds showed a CAC percentile above 75, and 42% above 90. Extensive calcification (CAC score> 400) was observed in 14% of patients. Cardiac CT quality was considered optimal in most patients and only 9% were deemed nondiagnostic. Lesions that originated stenosis≥25% were analyzed, resulting in a mean of 5.5 lesions per patient (median 4). Regarding plaque characteristics, only 29% of these lesions were considered “soft” or fibrous, whereas lesions with calcification constituted the majority, including mixed (26%) and purely calcified or “hard” plaques (45%). We found no association between the GRS profile and CAC score, the number of coronary stenosis assessed by CT, or multivessel disease by invasive coronary angiography.
There was an interesting trend between multivessel disease as assessed by CT and recurrent cardiovascular events (HR, 2.1; 95%CI, 0.9-4.8; P=.07), which was not detected with CAC score or multivessel significant stenosis assessed by invasive angiography.
Markers of extracellular matrix metabolismPatients with a more severe myocardial infarction, as determined by Killip-Kimbal class II or above, showed higher values of MMP-7 and TIMP-1 in the acute phase than those with Killip I (30.1±5.1 vs 26.6±1.9; P=.04 and 322±221 vs 166±72; P=.04, respectively). In addition, there was a significant negative correlation between left ventricular ejection fraction and MMP-7, MMP-10 and TIMP-1. However, we found no association between MMPs and other clinical variables indicating a more extensive atherosclerosis by calcium score, number of coronary segments with stenosis or with the GRS. In the multivariate Cox model, MMPs were not independent predictors of recurrent cardiovascular events.
DISCUSSIONThe major finding of our study is that the implementation of a GRS after an AMI in young nondiabetic patients may have clinical utility for the prediction of recurrent cardiovascular events independently of other clinical variables. Remarkably, the relevance of a moderate or high-risk GRS was limited to patients with high LDL-C, which acted as modifier. Conversely, among patients with high LDL-C, a poor genetic background could boost their recurrence risk compared with a more favorable genetic score. Therefore, patients with elevated LDL-C could benefit most from genetic testing, validating for the first time a hypothesis that remained unconfirmed.19 Hence, the possibility to detect individuals at higher risk represents an actionable goal to tailor more aggressive secondary therapy.
GeneticsIn our analysis, a well validated GRS composed of 11 genetic variants, including 8 SNPs and 1 haplotype was predictive of recurrent cardiovascular events during a 4.1 year follow-up after an AMI in young nondiabetic patients. A poor genetic background was associated with a higher risk of the primary endpoint, which was markedly increased when LDL-C levels were raised, a hypothesis that has been recently proposed.19 Inclusion of the GRS improved the C-statistic (ΔC-statistic=0.086) in a model adjusted for GRACE risk score and LDL-C. The population undergoing genetic testing had a high likelihood of genetic susceptibility for CAD, reflected in the almost 2-fold higher prevalence of most of the risk alleles variants analyzed compared with the general population of the same ethnicity. While the clinical usefulness of this GRS for prediction of incident CAD had already been tested, its use in secondary prevention was mainly unknown.
SNPs arising from large-scale GWAS are primarily related to atherosclerotic disease mediated through risk factors, and, to a lesser degree, directly to AMI.20 Pathophysiological findings underlying CAD and AMI differ, with a greater influence of thrombosis in AMI.2 This might partially explain the different results seen in primary and secondary prevention. The SNPs included in our study act either independently of classic risk factors or their mechanism was unknown.3
Studies evaluating the use of individual SNPs for the prediction of recurrent cardiovascular events after an ACS initially showed good predictive capacity but they usually lacked subsequent confirmation. In one study, 21 an individual SNP in the 9p21 locus was initially associated with recurrences after AMI, but a later meta-analysis excluded any significant influence for the risk of recurrence.22 Another single risk variant identified in the AB0 gene weakly improved a clinical risk prediction model for recurrences in a different study,23 but this finding has not been subsequently replicated.24
SNPs associated with CAD are common, and individually they only account for a small increase in risk. This has led to the development of GRS composed of multiple SNPs. Only a few studies have tested GRS for secondary prevention with conflicting evidence. Labos et al.25 were unable to find an association between a 30-SNP GRS and early recurrent events (< 1 year after ACS). Although these authors conclude that the etiology of early events may differ from that of later events, limiting the use of genetic testing, there are some possible explanations for this outcome: a) the variants that composed this GRS mainly covered genes associated with atherothrombosis, which is one of the main targets of drugs used after AMI; it is plausible that once specific therapy has been established, the variant-associated risk might be subjugated20; b) the GRS lacked a previous validation in the general population, and all the SNPs were derived from GWAS studies, which may account for only 10% of genetic susceptibility.2 In our study, we intentionally used a GRS previously validated for CAD but initially unrelated to risk factors. Mega et al.19 found an association between genetic risk categories based on a 27-SNP GRS and incident and recurrent events among patients included in statin clinical trials. The benefits of statins were greater in patients with intermediate or high genetic risk. These authors suggest that a high genetic burden should constitute a distinct focus of attention, since their risk of recurrences is increased and their benefit from therapy is greater. Our results are in line with these findings, and they replicate for the first time the ability of GRS to predict recurrent cardiovascular events linking the effect with dyslipidemia. Finally, Vaara et al.24 evaluated 2 GRS based on 47 and 153 SNPs respectively; whereas GRS47 showed some positive results, GRS153 did not, none of them were associated with the composite endpoint, and they did not substantially improve risk prediction models; these authors mention as a limitation the reduced number of young patients in whom the association might be stronger.
Several factors may have influenced the different outcomes: first, the heterogeneity of populations and variable follow-up; second, GRS composed of a higher number of variants cover a larger proportion of genetic predisposition, but they have consistently shown worse predictive ability3 and, finally, although most studies have used a composite outcome, they vary in their definition of recurrences, which limits comparisons.
Similar to prior studies, we found no association between a family history of CAD and GRS, which reflects the value of genomic data beyond self-reported family history.3
Cardiac computed tomographyCalcification and multisegment and/or multivessel disease were higher than previously reported in this population compared with other studies using invasive coronary angiography to characterize nonculprit lesions. This may reflect the fact that even in young patients with an acute clinical onset, subclinical atherosclerosis has a long-standing course. The clinical value of cardiac CT for patients with ACS is limited: while the number of coronary plaques showed a trend toward a higher risk of events, the calcium score did not. This probably reflects the usual pathway of ACS, with plaque rupture of lipid-rich nonstenotic lesions rather than chronic and calcified lesions. We found no association between the number of risk alleles and baseline coronary calcium score or number the plaques detected by CT in the first common description in the literature among patients with AMI.
Extracellular matrix metabolismMMPs are mediators of plaque rupture and atherothrombosis. They are increased after myocardial infarction and have been proposed as predictors of heart failure and adverse remodelling.26 In our study, MMP-7, MMP-10 and TIMP-1 were associated with a more severe myocardial infarction in the acute phase but did not predict left ventricular recovery or recurrences during follow-up.
The strengths of our study include a homogeneous population with a high likelihood of genetic predisposition, comprehensive clinical and biohumoral information, and complete follow-up. Cardiac CT with calcium score and plaque analysis offers an unprecedented vehicle for characterization of underlying disease linked to patients’ genetic profile.
LimitationsThis study has some limitations. First, because of the highly restrictive selection criteria, the number of patients is relatively low; selection was based on previous observations that younger individuals are more prone to have genetic contributors to their recurrence risk27; therefore the results may not be generalizable to the whole spectrum of ages, diabetic population, or different ethnicities. Second, we used a composite endpoint, similar to comparable studies, but exposed to include events not genetically-driven. Third, the high use of optimal medical therapy resulted in fewer follow-up events than expected.
CONCLUSIONSA GRS that combined 11 SNPs identified individuals at highest risk of long-term recurrences among young nondiabetic patients with myocardial infarction and improved clinical risk stratification models. Patients with high LDL-C may derive the greatest benefit from genetic testing. Cardiac CT detected a calcified substrate with numerous atherosclerotic plaques but had limited value in long-term risk prediction.
FundingThis study was supported by the Instituto de Salud Carlos III (PI12/0564, PI14/01152 and PI15/00667), the CIBERCV (CB16/11/00250), the Spanish Society of Cardiology (2015/Cardiología clínica) and Fundación Eugenio Rodríguez Pascual.
Conflicts of interestNone declared.
- -
Dozens of common genetic variants are associated with CAD, each leading to a small increase in risk. GRS have been generated as a summation of multiple loci to provide an estimate of individual-level risk. GRS have been positively tested in the general population to assess the risk of a first cardiovascular event. However, the usefulness of genetic risk markers for the prediction of recurrent events among patients with a prior myocardial infarction remains largely unknown.
- -
This is the first series in the Spanish population to study a multilocus GRS in a homogeneous group of young patients with a high likelihood of a genetic predisposition to recurrence who had already had a myocardial infarction. The implementation of the GRS was clinically useful for the prediction of recurrent cardiovascular events independently of other variables and improved clinical risk stratification models. This study serves as a proof of concept for the use of genetic tools in secondary prevention after a myocardial infarction.
Supplementary data associated with this article can be found, in the online version available at https://doi.org/10.1016/j.rec.2019.08.006