Keywords
ABDOMINAL AORTIC ANEURYSMS
The abdominal aortic aneurysm (AAA) is defined as an increase in the aortic diameter of more than 50% with respect to its original size. Characteristically, it affects men starting in the seventh decade of life. Its prevalence increases in our general population in parallel with the increase in life expectancy and the decrease in cardiovascular mortality. Its rupture is the major clinical consequence. Aneurysm rupture is associated with an overall mortality rate of nearly 90%.1
Rupture is avoided with early surgical resection and prosthetic replacement. However, the mortality with elective treatment is around 5%.2 More recently, the introduction of endovascular treatment has enabled elective and emergency treatment, with more promising initial results owing to the utilization of this less aggressive technique.3
Aneurysmal disease mainly affects older men and white smokers. In population-based studies, the prevalence of aneurysms measuring between 2.9 and 4.9 cm ranges from 1.3% in men aged 45 to 54 years to 12.5% in those aged 75 to 84 years,4 and the male-to-female ratio is 4:1. Rupture is a common cause of mortality in the general population, with 15 000 deaths per year in the United States; it is the fifteenth most common cause of death and ranks tenth among men over the age of 55.5
The purpose of screening for aneurysmal disease is to reduce the mortality and the health care costs associated with treatment for ruptured AAA. Ultrasound examination is highly sensitive and specific, is noninvasive and inexpensive. Scott et al,6 in a study involving the screening of 16 000 patients, reported a prevalence of AAA greater than 3.0 cm of 4.0% (7.6% in men and 1.3% in women) and a reduction of the incidence of rupture of 55%. However, given the low prevalence of patients in whom surgery is indicated on the basis of the diameter, representing 3.5% of those AAA measuring less than 4.5 cm and 0.83% of those ranging between 4.5 cm and 5.9 cm, in a series of 1700 individuals aged 65 to 74 years,7,8 some authors consider that the cost-effectiveness of screening in an unselected population to be poor.
Smoking9 and peripheral arterial disease10 have been associated with a higher prevalence of AAA, as has the existence of first-degree relatives with AAA. Under these circumstances, the prevalence ranges between 15% and 30%.11 Thus, screening would be reasonable in men over 60 years of age12 who smoke or present other associated factors, such as peripheral aneurysms and a first-degree family history of AAA.13
The most common location for an AAA is the infrarenal segment, with suprarenal involvement in 5% of the cases. The simultaneous presentation in other territories is a frequent occurrence, with thoracic, iliac or peripheral aneurysms being detected in 12%, 25%, and 3.5% of the patients, respectively. The femoropopliteal aneurysm can be accompanied by an AAA in up to 70% of the cases.13,14
Etiology
The etiology and pathogenesis of acquired aneurysmal disease are complex and have yet to be fully elucidated. Histologically, degeneration of the structural elements and remodeling of the media of multifactorial origin are observed, with evidence of chronic inflammation, destruction of the elastic lamina and smooth muscle depletion. Hereditary, atherosclerotic, infectious and inflammatory factors, as well as changes in the proteolytic activity of the aortic wall, have been proposed. The influence of biomechanical factors has also been related to the formation, growth and rupture of aneurysms.
There are a number of reports of the presence of a genetic predisposition, as aneurysmal degeneration associated with first-degree family history has been detected in up to 28% of the male patients.4 Familial aggregation does not appear to influence the rate of expansion, although it does seem to be related to an earlier age at onset.15 Polycystic kidney disease, an autosomal dominant disorder, has been associated with AAA,16 although hypertension (HT) and connective tissue diseases could be the underlying cause of the association between AAA and renal disease. Prockop17 identified a structural change in type III collagen in members of a family who had died due to ruptured AAA.
Although some authors have attempted to show that atheroma and aneurysmal degeneration are independent processes, the greater prevalence of smoking, HT, ischemic heart disease and peripheral arterial disease, as well as differences in lipoprotein concentrations,18 indicate the participation of atherosclerosis in the process. Markers of the extension of atherosclerosis, such as the presence of atheromatous plaques in the thoracic aorta, have been associated with a higher prevalence of AAA (14% vs 1.4%).19
Pathophysiology
The factors that trigger a response in the form of a chronic inflammatory process have yet to be fully defined. The presence of lymphocytic/monocytic inflammatory infiltrates and macrophages, with an increase in cytokines and other mediators, leads to an abnormal increase in the proteolytic activity in the extracellular matrix,19,20 with the destruction of elastin and collagen and an immune response to their degradation products. The participation of infectious agents has been proposed, as herpesvirus and Chlamydia pneumoniae have been detected in 30% to 50% of the cases of AAA, as have antibodies to Chlamydia.21,22 On the other hand, the depletion of smooth muscle attributed to the induction of cell apoptosis,23 together with the increase in the metalloproteases of the extracellular matrix, produce an abnormal remodeling that is unable to impede thinning and aneurysm expansion.
The biomechanical properties of the aorta are modified in relation to the structural changes and arterial geometry. Although Laplace´s law has been used to explain the relationship among surface tension, radius and arterial pressure (AP), this model is only applicable to cylindrical structures, while in AAA, the growth makes it necessary to adopt a spherical conformation. This change in morphology reduces wall tension with respect to that obtainable on the basis of said law.25 The maximum size and the risk of rupture, on the other hand, can not be considered to be synonymous in every case, since the composition of the wall and the morphology of the aneurysm are not uniform, and the points of greatest tension do not coincide with that of the greatest diameter located in points of inflection and transition. Likewise, the protective effect of the mural thrombus is another misleading concept, as we observe that its thickness is related to local ischemic phenomena in the underlying wall.26
Diagnosis
The incidental finding of an asymptomatic AAA during an examination of the abdomen for the study of other diseases is not uncommon: a pulsatile mass on abdominal palpation, calcified borders on plain abdominal radiograph or the identification of an aneurysm during the ultrasound examination of a patient undergoing a study of the prostate gland or magnetic resonance for spinal disease are the circumstances that most frequently lead to the diagnosis of an asymptomatic AAA.
However, there are certain subtle, nonspecific clinical signs that accompany a minority of AAA. Compression of the inferior vena cava (IVC) can present with lower extremity edema. When the urinary tract is affected, there can be lumbar pain, with urological symptoms. In inflammatory aneurysms (5% to 10%), fibrosis in neighboring structures (duodenum, IVC, right renal vein, ureter, or sigmoid colon) can be accompanied by abdominal or low back pain, with weight loss, increased erythrocyte sedimentation rate, and the presence of a perianeurysmal inflammatory halo in the tomographic study. Peripheral arterial embolization leads to acute or chronic ischemic episodes caused by recurrent embolization. Atheroembolism can present as blue toe syndrome. Finally, arterial infection resulting from hematogenous dissemination or proximity, accompanied by febrile syndrome and positive blood cultures in the context of compromised aortic wall integrity, are the clinicopathological characteristics of mycotic aneurysms.
Aneurysmal rupture is the most serious form of onset, and is life-threatening if not treated surgically. It occurs frequently in patients with undetected aneurysm. Thus, clinical suspicion is the key to avoiding the delay in treatment, one of the factors related to its high rate of operative mortality.27 In 2% to 3% of the cases, the clinical signs of rupture may be contained, with low back pain, hemodynamic stability and no perivascular hemorrhage. However, rupture most commonly presents in the form of retroperitoneal hemorrhage (90%) with circulatory collapse, which can be transient, and an increased abdominal circumference. Occasionally, the onset involves bleeding toward an adjacent organ, such as the intestine (1%), in which there can be self-limited gastrointestinal bleeding, with febrile episodes due to bacteremia; bleeding toward the territory of the iliocaval venous system (1%), with venous hypertension, heart failure, pulmonary embolism, lower extremity edema or nephrotic syndrome; or intraperitoneal rupture (5%), with fatal consequences if not treated immediately.
The sensitivity of the clinical diagnosis of AAA is limited for diameters of less than 5 cm in patients whose anatomy is little favorable for abdominal palpation. Plain abdominal radiography can reveal indicative arterial calcification.
Ultrasound is an accessible, noninvasive technique, with a sensitivity of 92% to 99% and a specificity of 100%,28-30 that is valid for the measurement of diameters and the mural thrombus. In rupture, signs such as the presence of fluid collections can be identified. Despite the limitations of the juxtarenal and suprarenal and iliac studies in unselected patients, it is the exploration of choice for follow-up.
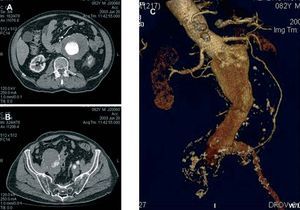
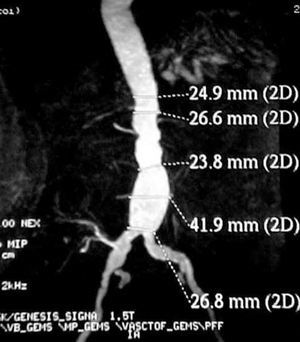
Contrast-enhanced computed tomography (CT angiography) provides high-resolution images and the possibility of 3-dimensional reconstruction. It is the technique of choice in the diagnosis and the preoperative study. The aortoiliac anatomy, the characteristics of the wall (inflammation, calcification, signs of rupture, thrombus), the visceral vessels and anatomical variations (horseshoe kidney, left renal vein, or left IVC) can be evaluated in detail (Figure 1). The disadvantages are the radiation and the use of a contrast agent. Contrast-enhanced magnetic resonance (MR) has the same properties as CT angiography, but eliminates the risk of nephrotoxicity and ionizing radiations (Figure 2). The degree of stenosis in visceral vessels may be overestimated, it does not detect calcifications and it produces artifacts in individuals with steel implants. However, in the follow-up of endoprostheses made of nitinol, MR angiography has been found to be useful in the detection of leaks.31-33
Figure 1. Axial images of abdominal aortic aneurysm and both iliac arteries (A and B). Detailed 3-dimensional reconstruction of the anatomy.
Figure 2. Image of an abdominal aortic aneurysm obtained with magnetic resonance angiography in a renal transplant recipient (right iliac artery). (Image courtesy of Dr I. García of Hospital Valdecilla, Santander, Spain.)
Conventional arteriography can also be performed in the preoperative evaluation of patients with suspected visceral and peripheral atherosclerotic lesions when it is necessary to examine the renal vessels, or to assess the meandering mesenteric artery in patients with clinical signs of intestinal ischemia or with previous gastrointestinal surgery. Given that it only provides information on vessel lumen opacification, this technique is not valid for obtaining measurements.
Prognosis
In the natural history of AAA, their growth determines their progression toward rupture. This complication is common in aortoiliac and visceral abdominal aneurysms, in contrast to those involving peripheral territories, in which ischemic disease secondary to thrombosis and/or embolization predominates.
The growth rate differs depending on the initial diameter at the time of diagnosis. Thus, the annual growth rate in AAA measuring less than 4.0 cm ranges between 1 mm and 4 mm. In AAA of 4.0 cm to 6.0 cm, the annual growth is between 4 mm and 5 mm and, in larger AAA, the growth rate can be up to 8 mm/year.34 The diameter is considered to be the most significant predictive factor of rupture,35 with an annual risk of nearly zero for aneurysms of less than 4 cm. The annual risk of rupture increases to between 0.5% and 5% in AAA measuring 4 cm to 5 cm, to between 3% and 15% in those of 5 cm to 6 cm, to between 20% and 40% in those of 7 cm to 8 cm and reaches 50% in AAA larger than 8 cm.
The capacity of surgical treatment to interfere with the natural history of small aneurysms (between 4 cm and 5.4 cm) has been studied in 2 prospective, randomized studies (the Veterans Affairs Aneurysm Detection and Management [ADAM] Study and the UK Small Aneurysm Trial).34,35
The benefits of early treatment of aneurysmal disease were not determined due to the low incidence of rupture of visceral and peripheral atherosclerotic aneurysms, 0.6% and 3.2%, respectively. However, a higher risk of rupture was observed in 17% of the women in the UK Small Aneurysm Trial, with an overall mortality rate of 14% versus 4.6% in men. In the study of the behavior according to size, 81% of the patients in the ADAM study in whom surgical treatment was indicated because of the growth of the AAA presented an initial diameter of 5.0 cm to 5.4%. The beneficial effects of endovascular treatment of small AAA are unknown. Thus, 2 prospective, randomized trials are underway to study this question: the CAESAR trial in Europe and its equivalent in the United States, the PIVOTAL study.36,37
Other factors related to rupture are HT,38 chronic obstructive pulmonary disease (COPD), smoking and family history. The influence of atmospheric pressure on the incidence of rupture has also been reported.39
Treatment
Pharmacological Considerations in the Treatment of Abdominal Aortic Aneurysms
At the present time, there is no scientific proof of the influence of drug treatment on the control of aneurysm growth. The increasing knowledge of the etiology and pathogenesis of the process provides new therapeutic perspectives that will need to be evaluated in future studies.
The control of atherosclerotic risk factors should be part of the basic treatment of these patients, beyond their potential effect on the natural history of AAA, because of the reduction in cardiovascular morbidity and mortality. The association between COPD and smoking and the growth and rupture of small aneurysms has been reported. Hyperlipidemia and poor control of HT, related to AAA, are also modifiable factors. Some authors have indicated that the use of statins is potentially beneficial due to their interference in the aforementioned molecular phenomena.
In addition, nonsteroidal antiinflammatory drugs (NSAID) (indomethacin)40 and tetracyclines (doxycycline)41 have been reported to interfere with the inflammatory process in patients with small aneurysms, in whom a reduction in the rate of growth with respect to controls has been observed, probably mediated by the decrease in the levels of matrix metalloproteases (MMP-9).
Indications for Surgical Treatment
In practice, due to the generally asymptomatic presentation of AAA, the aortic diameter is usually the major clinical criterion for the indication for treatment. After annual follow-up examinations in AAA smaller than 4.0 cm, performed at 6-monthly intervals in those measuring 4.0 cm to 4.9 cm, surgical treatment is recommended for AAA of 5 cm to 5.5 cm in men and of 4.5 cm to 5.0 cm in women. Some authors take into consideration a diameter of the aorta 2-fold greater than that of the previous healthy aorta. Other absolute indications for treatment are the presence of an embolic episode, disabling iliac occlusive disease, coexistence of iliac aneurysm, low back or abdominal pain attributable to the AAA and a growth rate of more than 5 mm/year. When the diameter is smaller, saccular morphology and nondisabling occlusive disease are relative indications.2,4
Conventional Treatment
Since its introduction by Dubost in 1951, the treatment of choice for AAA is the replacement of the aneurysmal segment with an aortoaortic or bifurcated prosthetic graft, depending on whether the iliac territory is affected by atherosclerotic aneurysm or occlusive disease. The classical approaches involve midline laparotomy or the retroperitoneal approach via left flank. Although some authors claim that the retroperitoneal incision provides better access to the suprarenal aorta and is associated with a lower incidence of respiratory complications,42 the right lateral decubitus position makes it more difficult to gain access to structures on the right side, such as right iliac or renal artery. Moreover, retroperitoneal approaches are associated with a higher incidence of incisional hernia.
The mortality rate in elective treatment of AAA is approximately 5%. In a metaanalysis carried out by Steyerberg et al,43 a plasma creatinine level over 1.8 mg/dL, congestive heart failure, electrocardiographic ischemia, lung disease, age and female sex were identified as risk factors. The morbidity and mortality associated with this procedure are linked to aortic narrowing and visceral territory ischemia. In the former, the cardiac status should be assessed preoperatively44; in the latter, renal and intestinal revascularization should be considered in the event the renal, mesenteric and/or hypogastric arteries are compromised.
The major complications of conventional treatment of AAA are cardiologic (15%) such as myocardial infarction (2% to 8%), respiratory (8% to 12%) such as pneumonia (5%), renal failure (5% to 12%) with hemodialysis (1% to 6%), venous thrombosis (8%), wound infection (over 5%), bleeding (25%), extremity ischemia (1% to 4%), and, to a lesser extent, stroke, urinary tract lesion, colonic ischemia, spinal cord ischemia, thrombosis, and graft infections.43,45 In the latter case, systemic antibiotic prophylaxis and covering the prosthetic material with the aortic sac are the main measures for preventing the late development of aortoenteric fistulas, which are associated with a mortality rate similar to that of rupture.
Survival at 5 and 10 years is 70% and 40%, respectively. It improves significantly in the absence of heart disease and HT. The long-term survival rate in cases of rupture is lower due to associated comorbidities.
Endovascular Treatment
Endovascular treatment has been increasingly employed since the initial clinical experiences of Parodi in 1991.46 Aneurysm exclusion is achieved by means of endograft implantation. With the consolidation of endovascular treatment, the degree of complexity of the aneurysms treated has increased because of the versatility of the endograft, making it possible to place aortouniiliac grafts, bifurcated grafts, grafts with suprarenal fixation and fenestrated grafts, or use hybrid techniques in which aneurysm exclusion is combined with surgical visceral revascularization. Although this approach was initially indicated only in those patients in whom conventional surgery was highly risky, there are many groups that advocate endovascular AAA repair (EVAR) as the first option. When the anatomy is favorable, the advantages are significant: a high rate of technical success (96% to 100%), avoidance of laparotomy, bowel manipulation and aortic clamping, a lower incidence of postoperative ileus and need for transfusion, better control of postoperative pain and shorter intensive care unit and overall hospital stays.47 Above all, the rate of perioperative mortality is lower, as has been demonstrated in recent prospective, randomized trials involving patients at low surgical risk.48-50 The reduced invasiveness has made its application in aneurysm rupture possible, with promising results.51
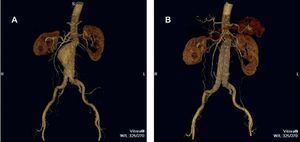
The purpose of EVAR is to provoke the thrombosis of the excluded sac, the reduction of the aneurysm diameter and, finally, the elimination of the risk of rupture (Figure 3). However, specific complications (leaks, migration, material fatigue, graft occlusion, infection) (Table 1) require indefinite patient follow-up and are frequently the cause of reinterventions. It is precisely the reinterventions, mostly involving endovascular procedures associated with a low mortality rate, that limit the widespread use of EVAR. However, future technological developments will minimize this drawback.52,53
Figure 3. Image of an abdominal aortic aneurysm obtained by computed tomography angiography with 3-dimensional reconstruction (A). The results of exclusion by means of endoprosthesis implantation can be observed (B).
Follow-Up of Endovascular Treatment
Conventional surgery requires long-term clinical and instrumental follow-up, since conventional prostheses can produce complications, such as infection, thrombosis, pseudoaneurysms, or aortoenteric fistula. Given its innovative nature, the fatigue of the materials involved in the manufacture of the endoprosthesis and the fixation, which is less active than that provided by the surgical suture, EVAR requires a closer and more intense clinical and instrumental follow-up.52 Computed tomographic angiography is the technique of choice for the preoperative study and the detection of most of the complications. To limit radiation and the use of contrast agents, which have been related to a more marked deterioration of renal function than that produced by the intervention itself, the indications for this technique may be selective, after conventional radiography in 4 projections and validated Doppler ultrasound (Table 2). Radiography enables the evaluation of the integrity of the metallic structure, placations, disconnections and graft migration. Doppler ultrasound determines the aortic diameter, graft patency, and the presence of leaks, with a sensitivity of 95% and specificity 97%.53 However, there are limitations related to the examiner and its practicality in obese patients. Magnetic resonance angiography has been found to have a sensitivity of 94% for the detection of type II leaks, is less nephrotoxic and is associated with fewer artifacts with the introduction of nitinol devices.33
In general terms, owing to its invasiveness and the relatively lower sensitivity in the identification of type II leaks, arteriography is a complementary technique, confined to the treatment of leaks in the surgical setting.
RENOVASCULAR DISEASE
Renal artery stenosis (RAS) is a common finding in atherosclerosis related to HT and deterioration of renal function (RF). However, the situation of patients with RAS is complex. Multiple factors play a role in the pathogenesis of HT and renal failure. Consequently, renal revascularization has not been found to be effective in every case, despite the increasingly widespread performance of percutaneous procedures.
Renal artery stenosis is a common finding in patients with atherosclerosis and an infrequent cause of secondary HT. Its prevalence is difficult to determine. Holley et al54 reported an incidence of 22.4% in a series of 295 autopsies. In individuals over 65 years of age, Doppler ultrasound revealed an incidence of stenosis greater than 60% of 6.8% (9.1% in men and 5.5% in women),55 although autopsy studies have documented the presence of RAS in around 30% of subjects over the age of 60.54 Atherosclerosis in other territories increases the prevalence of RAS, which can reach 20% in patients with coronary heart disease56 or 33% to 50% in those with peripheral arterial disease.57 Bilateral involvement has been reported in up to 44% of the patients with RAS.58
In hypertension registries, renovascular disease is the cause of less than 1% of unselected cases, and occurs in 5% of the patients with other signs of atherosclerosis and in 7% to 17% of the patients with a diastolic arterial pressure over 110 mm Hg or refractory HT.59
Etiology
Atherosclerosis is implicated in over 60% of the cases of RAS. Fibromuscular dysplasia (FMD) can be the underlying cause in up to 30%, while the remaining causes (actinic damage, Takayasu´s disease, dissection, neurofibromatosis type 1) can account for up to 10%.59
Pathophysiology
There are considered to be 2 models of renovascular HT. In unilateral RAS, HT is secondary to increased plasma renin activity and is mediated by angiotensin II due to the reabsorption of sodium produced following activation of the renin-angiotensin-aldosterone system. In bilateral RAS or in patients with only 1 kidney, the absence of a contralateral kidney impedes the excretion of sodium and of the volume retained. Thus, the plasma renin activity is reduced and the process becomes dependent on the volume.60 Other pressor mechanisms have been proposed in renovascular HT, including sympathetic nervous system activity, activation of oxidative stress pathways, endothelial factors, and prostaglandins.61
There is a relationship between the progression of RAS and the deterioration of renal function.62 However, poststenosis ischemia is just one of the factors identified in the pathogenesis of renal failure in the context of atherosclerotic renal artery disease, which involves HT, atherosclerosis of the renal parenchyma, hyperlipidemia and atheroembolism observed in cases of RAS.63-65 The degree of stenosis and the severity of renal failure are not synonymous,66 since 10% of the metabolic activity of the kidney is provided for by the renal blood flow.67
In cases of bilateral lesions or in patients with only 1 kidney, the volume expansion produced by the reduction in natriuresis, as well as the decompensation of left ventricular function in association with the elevation of the arterial pressure during this increase in blood volume,68 can trigger recurrent signs of congestion disproportionate to the degree of ventricular systolic dysfunction and episodes of refractory angina. Ventricular hypertrophy can be mediated by mechanisms associated with aldosterone69 and, for the same blood pressure level, can be more serious in renovascular HT than in essential hypertension.
Diagnosis
The profile of the patient with RAS secondary to atherosclerosis is that of an older hypertensive man whose hypertension worsens in the context of an intercurrent vascular episode.70 The presence of RAS is not synonymous with renovascular disease. Thus, the identification of the indicative clinical features is important for correct diagnostic planning and therapeutic approach.
An age at presentation of HT of over 55 years is typical of the atherosclerotic patient, in contrast to HT in individuals under the age of 30, generally women, which is characteristic of FMD. Sudden and persistent worsening of known HT and its refractoriness to treatment with 3 or more antihypertenstive agents, including a diuretic, are clinical indicators of renovascular origin. Malignant HT with evidence of organ damage such as acute renal failure, acute decompensation of chronic congestive heart failure, the development of a new neurological or visual disturbance and or advanced retinopathy (grade III/IV) and a history of atherosclerosis at other sites are also indicative of a renovascular origin.70
In vascular HT, during arterial pressure (AP) control, a lability has been reported in the AP, secondary to the increase in sympathetic activity, with an absence of the nocturnal fall and episodes of flushing and autonomic instability, which are also indicative of pheochromocytoma.71
Ischemic renal failure is suspected in cases of de novo renal failure or deterioration of renal function following administration of angiotensin converting enzyme (ACE) inhibitors or angiotensin II receptor antagonists (angiotensin receptor blockers [ARB]). Unexplained renal atrophy or a difference in pole diameter of more than 1.5 cm associated with the deterioration of renal function or in cases of unexplained renal failure, including those of patients receiving replacement therapy (hemodialysis or transplantation), is also suspicious.
With regard to the cardiac manifestations, a prevalence of renovascular disease of up to 30% has been observed in patients admitted to the hospital with recurrent circulatory congestion or with episodes of refractory angina,72 that remit after renal revascularization.73 Thus, the identification of RAS by means of arteriography of renal ultrasound may be justified during the examination of patients with multivessel coronary artery disease or in association with peripheral artery disease.74
The search for RAS is justified when, in addition to a reasonable suspicion, there is a potential benefit in the treatment of AP, the renal function or the cardiovascular status. The diagnosis of renovascular disease is based on a correct clinical selection of the patients, the identification of the stenosis and functional tests.
Renal Arteriography
Arteriography is the reference test for the diagnosis of RAS. Due to its invasive nature, it can not be employed as a scanning technique. As the treatment of lesions diagnosed incidentally is controversial,74,75 the performance of a renal study during aortography is based on the criteria of the examiner. The morbidity and mortality associated with manipulation and the administration of the contrast agent is low. The incidence of contrast-induced acute renal failure is less than 3% in nondiabetic individuals with normal renal function, 5% to 10% in diabetics with normal renal function, 10% to 20% in patients with renal failure and can reach 20% to 50% in patients with both risk factors.76,77 At present, there is no single effective strategy for the prevention of contrast-induced renal dysfunction in patients with risk factors, in whom the use of nonionic contrast agents, hydration, oral N-acetylcysteine or hemofiltration has been proposed.
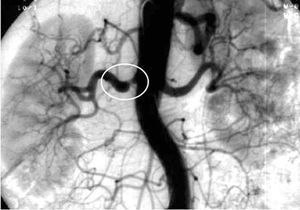
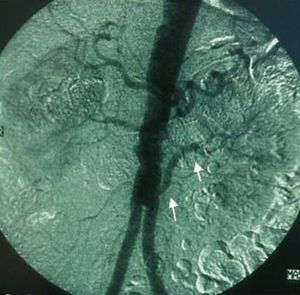
Thus, arteriography is indicated in those cases in which noninvasive studies are equivocal or there is high clinical suspicion and concomitant angiography must be performed (Figure 4).
Figure 4. Renal artery angiography showing marked stenosis in left renal artery (white circle) with mild poststenotic dilatation.
Renal Doppler Ultrasound
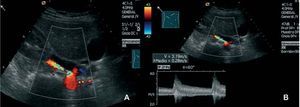
Doppler ultrasound has a sensitivity of up to 96% and a specificity of up to 98%.78 Although it does not enable direct functional assessment, it provides hemodynamic and sonographic information about the renal artery, the state of the parenchyma and renal anatomy, enhanced, unlike arteriography, with the use of ultrasound contrast agents79 (Figure 5). Although some authors propose it as the study of choice, it is an examiner-dependent technique that may prove impracticable in up to 20% of selected patients.61
Figure. 5. A: color Doppler ultrasound image of the stenosis depicted in Figure 4. B: increase in Doppler velocities in the region of the stenosis.
The assessment of the renal parenchyma can be carried out indirectly in the hemodynamic study by determining the resistance index (RI), which, when over 0.80, is indicative of small-vessel disease and parenchymal fibrosis. It has been postulated that these values are specifically associated with a deterioration of renal function or death, with levels of sensitivity, specificity, positive predictive value and negative predictive value of 64%, 98%, 92%, and 91%, respectively, versus 88%, 91%, 73%, and 97% for proteinuria over 1 g/day, and 83%, 96%, 86%, and 95%, respectively, for a creatinine clearance greater than 40 mL/min.80 The RI has also been employed to predict the response to revascularization in RAS. In a study of 38 patients who underwent percutaneous transluminal angioplasty (PTA) to treat RAS, there were 35 with a RI over 0.80 in whom revascularization was not accompanied by improvements in renal function, blood pressure or in the survival of the affected kidney. Thus, this index is considered to predict the failure of the technique.81
Contrast-Enhanced Computed Tomography and Magnetic Resonance
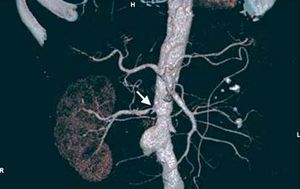
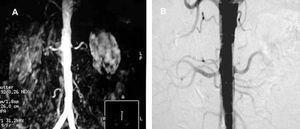
Contrast-enhanced multislice CT (CT angiography) and contrast-enhanced MR (MR angiography) assess aortic involvement, the bilaterality of the lesions and the presence of accessory vessels, which are not detected in the ultrasound study or, of course, in the functional studies. Renal size, renal anatomy and its variants (for example, horseshoe kidney) and the filtration rate can be assessed by means of both techniques (Figure 6). The cortical thickness obtained with CT is of value for estimating parenchymal damage.82 The sensitivity and specificity of CT angiography are 92% and 99%, respectively.83 The utilization of ionizing radiation and iodinated contrast agents are the most important limitations to its use for scanning, especially in patients with functional deterioration. The sensitivity and specificity of MR angiography are similar, ranging between 90% and 100% and between 76% and 94%, respectively. In contrast to CT angiography, ionizing radiations are not utilized and the gadolinium employed as contrast agent is less nephrotoxic. However, there are limitations for intrastent stenosis or nonostial lesions, such as FMD; it also tends to overestimate the lesions and provides no information on their calcium content84 (Figure 7).
Figure 6. Computed tomography angiography in a patient with diffuse atherosclerosis of both iliac limbs, abdominal aortic aneurysm, left renal artery occlusion and severe right renal artery stenosis (arrow).
Figure 7. A: view of critical left renal artery stenosis using magnetic resonance angiography. B: verification of a false positive by means of digital angiography.
Functional Study: Radioactive Isotope Renogram
In RAS, functional studies present shortcomings with respect to the diagnosis and prognosis that limit their use as scanning techniques. When the treatment of RAS represents a priori an immediate expected benefit, as in severe HT presenting as pulmonary edema, these studies can be obviated.61
Radioactive isotope renogram with captopril provides information on the renal size, perfusion and excretory capacity. A delay in the maximum activity, asymmetry in the peak activity of each kidney, prolonged cortical retention of the contrast medium or a decrease in the rate of glomerular filtration of the ipsolateral kidney after captopril administration are indicative of RAS.85 The diagnostic value is variable, with a sensitivity of 85% (45% to 94%) and a specificity of 93% (81% to 100%), which decrease to 74% and 59%, respectively, when compared with angiography.70 However, in hypertensive individuals without RAS, asymmetry due to parenchymal disease may be observed in tracings with radioactive isotopes, the lateralization may not be evidenced in bilateral stenosis or it may be difficult to identify RAS in the single kidney.86
Measurement of Plasma Renin
The stimulation of the diseased kidney with a diuretic or, more commonly, a vasodilator (captopril) is followed by an increase in the plasma renin activity.87
The determination of plasma renin by means of the captopril test has a sensitivity of 61% and a specificity of 86% for the detection of renovascular disease. However, since the discontinuation of antihypertensive therapy 2 weeks before the examination is recommended, this may complicate treatment in hypertensive patients, in whom calcium antagonists or alpha-1-adrenergic blockers can be employed.70,87
Selective catheterization of the renal vein can counteract the limitation of the previous test in the identification of the diseased side. The lateralization of the test is associated with a favorable prognosis in terms of blood pressure response to revascularization in 90% of the cases, whereas when lateralization is not present, the prognostic value is minimal (50%).61 However, in the case of renal artery occlusion, this test could prove useful in the identification of a hypertensive kidney requiring nephrectomy.88 The invasiveness of the study and the need to discontinue antihypertensive therapy may limit its use.
In general, functional tests do not provide a prognosis in terms of cure since it is not possible to quantify the importance of renal parenchymal lesions or the interferences of nonrenovascular hypertension or previous deterioration of the renal function.
Prognosis
Renal artery stenosis is progressive and leads to the deterioration of renal function. Caps et al89 reported that stenosis had worsened by 35% at 3 years and by 51% at 5 years after a follow-up study involving ultrasound examinations at 6-month intervals in 170 patients (295 kidneys), with 9 cases (3.1%) of occlusion. Multivariate analysis identified diabetes mellitus, HT and severe stenosis as independent factors associated with progression.36 Other authors have observed the risk of occlusion in up to 39% of lesions greater than 75%.90
Renal artery stenosis has been related to end-stage renal failure (ESRF) in 15% of the patients over 50 years of age on hemodialysis.91 However, the percentage of patients with RAS with irreversible functional deterioration is quantitatively lower. In a series of patients in which there were 164 cases of stenosis greater than 50%, ESRF was identified as the cause of death in 5% of the 44 patients who died over a 7-year of follow-up period, while in 75%, the cause was a cardiovascular event.92 This demonstrates the importance of RAS as a predictor of cardiovascular mortality.93,94 In an analysis of 3987 coronary angiograms, the survival at 4 years, studied in relation to RAS, was 89% for stenosis of less than 75% and 57% in patients with stenosis greater than that (59% for unilateral disease and 47% for bilateral disease).93 In a series of 258 cases of RAS treated by stent revascularization, the deterioration of the glomerular filtration rate, observed in 84.5% of the patients prior to revascularization, was identified as an independent factor associated with cardiovascular morbidity and mortality.94
Treatment
Medical Treatment
The conservative treatment of RAS involves the treatment of atherosclerosis. Smoking cessation and antihypertensive and lipid-lowering therapies can limit the course of renal atherosclerosis.
The ACE inhibitors, ARB, and calcium antagonists are indicated in the treatment of unilateral RAS-Beta blockers have also been shown to be effective in the treatment of HT associated with RAS.70
The interference of the ACE inhibitors in the renin-angiotensin-aldosterone system regulates vascular tone and the fluid-electrolyte balance that cause hypertension, while it has a beneficial effect on proteinuria (in diabetic and nondiabetic subjects), left ventricular hypertrophy and congestive heart failure. The use of ACE inhibitors improves survival in patients with renovascular HT.95 However, in bilateral stenosis and in patients with only 1 kidney, treatment with ACE inhibitors can be related to deterioration of renal function, with an incidence of 6% during the first year of treatment, which is reversible in around 90% of the cases.96 This effect may be secondary to the decrease in glomerular filtration produced by ACE inhibitors and ARB, to intercurrent states of volume depletion or to the use of vasodilators. Thus, the use of vasodilators and diuretics, as well as volume management, in patients treated with ACE inhibitors should be done with care. Likewise, it may be necessary to discontinue ACE inhibitors in the case of an increase in creatinine levels by more than 20% or by 0.5 mg/dL, a decrease in glomerular filtration rate by more than 30% or the detection of renal atrophy.61
Indications for Revascularization (Table 3)
The purpose of revascularization for renal artery atherosclerotic stenosis is to achieve HT control, preservation of renal function and treatment of the hemodynamic impact of volume expansion. In asymptomatic stenosis, the benefit of revascularization in unilateral lesions has not been well established. Although HT is rarely cured, an improvement in blood pressure and/or the reduction of the need for drug intake can be achieved. In FMD, the control of AP is the major indication, and is achieved in 90% of the cases. Renal function may improve or become stabilized, especially in the cases of a recent increase in plasma creatinine, decrease in glomerular filtration rate in the context of treatment with ACE inhibitors or ARB and preservation of renal diastolic blood flow (low RI). In contrast, the presence of proteinuria over 1 g/24 h, renal atrophy, parenchymal lesion and arteriolar disease, as well as potential atheroembolism secondary to the endoluminal procedure, have been related to the failure of the technique. The reduction in renin production decreases that of angiotensin and aldosterone, which improves vasoconstriction and volume expansion, and restores the sensitivity to diuretics. There is a notable postrevascularization functional improvement in congestive heart failure and RAS97,98 (Table 4).
The combination of analytical, histological, hemodynamic, and morphological factors can help to determine the postrevascularization course.99
Percutaneous Techniques
Despite the fact that there are numerous series dealing with percutaneous revascularization, there are no randomized studies demonstrating the superiority of percutaneous revascularization with respect to medical treatment in unselected patients. The metaanalysis carried out by Nordmann and Logan, involving 210 participants in 3 randomized studies, showed a weak tendency to lower the AP, especially in cases of refractory HT and bilateral RAS, and drug requirements in patients treated percutaneously. However, it was not possible to demonstrate the superiority of angioplasty with respect to cases in which antihypertensive therapy was effective, nor were changes in renal function observed. Other authors have reported that the previous deterioration of the renal function is related to the absence of a net change in the glomerular filtration rate and postrevascularization renal function.61
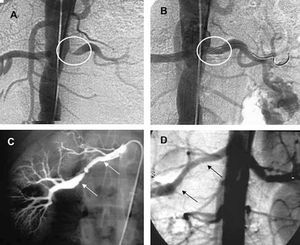
Percutaneous revascularization is the technique of choice in RAS secondary to FMD, with the use of the stent for the treatment of suboptimal dilatations (Figure 8). Restenosis is less common than in atherosclerosis, with an incidence of 8% in a series of 85 cases after a mean follow-up of 15 months.101 In atherosclerosis, the lesions are mostly located in the ostia, with greater elastic recoil. The use is associated with a higher incidence of surgical success, which can reach 98%, and restenosis is less common than in simple angioplasty (17% vs 26%, respectively).102
Figure 8. Percutaneous treatment of renal stenosis (A) with angioplasty and balloon-expandable stent (B) and fibromuscular dysplasia (C) by means of simple balloon angioplasty (D).
Van de Ven et al103 treated 85 patients who were randomly assigned to undergo angioplasty with or without stent. Although the rate of complications was similar and the rate of primary patency at 6 months was higher in patients who had undergone stent placement (75% vs 29%, respectively), there were no differences between the 2 groups with respect to AP control and renal function.103
Although the rate of complications is low, bleeding, vascular lesion, local embolization with segmental infarction, systemic atheroembolism, aortic and renal dissection, stent migration and thrombosis can develop, compromising renal viability.104 At the present time, antiembolic protection systems are still under development and, thus, protection systems that are not specific for the renal artery are being employed.
Surgical Treatment
Surgical treatment is indicated in the concomitant repair of the aorta due to aortoiliac or aneurysmal occlusive disease, adverse anatomy or involvement of the complex renal arteries (aneurysms, polar arteries, segmental artery lesions), failed percutaneous revascularization or the impossibility of treating restenosis by percutaneous techniques. Revascularization can be carried out by means of endarterectomy (renal or transaortic), aortorenal bypass or extraanatomic bypass (hepatorenal or splenorenal), reimplantation, autotransplantation, or ex vivo surgery for the repair of segmental arteries. The hypertensive kidney with renin hypersecretion may require nephrectomy. The mortality observed in surgical treatment is influenced by the comorbidities associated with atherosclerosis, as well as the complexity of the reconstruction, and is higher in combined aortorenal surgery (Figure 9). Hansen and Dean105 reported an operative mortality of 5.3% in patients undergoing combined surgery, as compared to 1.7% and 0.7% in procedures limited to the renal artery and aorta, respectively. Weibull et al106 randomized 58 patients to surgical or percutaneous treatment, with equivalent results in terms of survival and blood pressure control. In Spain, the progressive disuse of revascularization surgery in favor of percutaneous procedures has resulted in the circumstance that the former is being performed in increasingly fewer centers.
Figure 9. Example of left aortorenal bypass (arrows) in an aortobifemoral revascularization procedure to treat Leriche syndrome.
ABBREVIATIONS
AAA: abdominal aortic aneurysm
ACE: angiotensin converting enzyme
ADAM: Aneurysm Detection and Management Study
AP: arterial pressure
ARB: angiotensin receptor blockers (angiotensin II receptor antagonists)
CAESAR: Comparison of Surveillance versus Aortic Endografting for Small Aneurysm Repair Trial
COPD: chronic obstructive pulmonary disease
CT: computed tomography
ESRF: end-stage renal failure
EVAR: endovascular aneurysm repair
FMD: fibromuscular dysplasia
HT: hypertension
IVC: inferior vena cava
MMP-9: matrix metalloproteinase 9
MR: magnetic resonance
NSAID: nonsteroidal antiinflammatory drugs
PTA: percutaneous transluminal angioplasty
RAS: renal artery stenosis
RF: renal function
RI: resistance index
Section Sponsored by Laboratorio Dr. Esteve
Correspondence: Dr. V. Riambau.
Cirugía Vascular. Hospital Clínic.
Villarroel, 170. 08036 Barcelona. España.