Adipose tissue has long been considered an energy storage and endocrine organ; however, in recent decades, this tissue has also been considered an abundant source of mesenchymal cells. Adipose-derived stem cells are easily obtained, show a strong capacity for ex vivo expansion and differentiation to other cell types, release a large variety of angiogenic factors, and have immunomodulatory properties. Thus, adipose tissue is currently the focus of considerable interest in the field of regenerative medicine. In the context of coronary heart disease, numerous experimental studies have supported the safety and efficacy of adipose-derived stem cells in the setting of myocardial infarction. These results have encouraged the clinical use of these stem cells, possibly prematurely. Indeed, the presence of cardiovascular risk factors, such as hypertension, coronary disease, diabetes mellitus, and obesity, alter and reduce the functionality of adipose-derived stem cells, putting in doubt the efficacy of their autologous implantation. In the present article, white adipose tissue is described, the stem cells found in this tissue are characterized, and the use of these cells is discussed according to the preclinical and clinical trials performed so far.
Keywords
Adipose tissue is one of the most abundant human tissues. It constitutes between 15% and 20% of the body weight of men and between 20% to 25% of that of women and is widely distributed throughout various body regions. This specialized tissue is of mesenchymal origin, consisting of a combination of white adipose tissue (WAT) and brown adipose tissue, with each tissue type showing distinct functions, morphologies, and distributions. In both tissues, the predominant cell is the adipocyte, comprising between one- and two-thirds of the total, and the remaining tissue is composed of different types of cells constituting the stromal vascular fraction (SVF).
White Adipose TissueAlthough WAT is distributed throughout the body, its principal deposits are subcutaneous, where it acts as an energy storage system, and in the visceral or intra-abdominal region, where it protects against possible trauma. The 2 tissues show different adipokine expression profiles,1 metabolic functions,2 vascular density, and innervation. Visceral adipose tissue shows a higher angiogenic potential and more acute inflammatory profile than subcutaneous tissue.3 The accumulation of subcutaneous adipose tissue represents a physiological response to situations of excessive intake and low energy expenditure (physical inactivity), acting as an “energy sink”. Individuals with peripheral obesity (subcutaneous distribution) do not show the characteristic medical complications of obesity. In contrast, increased visceral adipose tissue (central obesity) is associated with a state of hyperglycemia, hyperinsulinemia, hypertriglyceridemia, hypercholesterolemia, reduced circulating levels of high-density lipoproteins, decreased glucose tolerance, increased apolipoprotein B-rich lipoproteins, and hepatic steatosis. All of these conditions are characteristics of insulin resistance syndrome, which increases the risk of the development of type 2 diabetes mellitus.4 Currently, waist size is an important diagnostic component of metabolic syndrome and has been identified as an independent risk factor for other diseases, such as cardiovascular diseases, stroke, hypertension, and nonalcoholic fatty liver disease.5–7
The main function of WAT is to regulate the energy homeostasis of the body, which is under the control of the nervous and endocrine systems. In times of caloric excess, adipose tissue stores fat in the form of triglycerides. These lipids are then released into the blood in times of energy demand to be used as an energy source in other tissues, such as the liver, kidneys, skeletal muscle, and myocardium.8 However, WAT is currently considered a multifunctional organ because, besides its energy function, it acts as an endocrine organ and as a reservoir of mesenchymal stem cells (MSCs).
Composition of White Adipose TissueWhite adipose tissue consists of mature adipocytes and intercellular tissue or SVF. Adipocytes, the most abundant cells in adipose tissue, contain a single large cytoplasmic vacuole that mainly stores triglycerides and cholesterol esters. Depending on nutritional status, adipocytes can alter their size to between 25 μm and 200μm. Adipocytes contain the machinery necessary for lipid metabolism (Figure 1).9 These pathways can be altered by an increase in weight, triggering insulin resistance syndrome. Indeed, fatty acids not only show an energy function, but also act as regulatory signals of the gene expression of proteins involved in lipid metabolism,10 favor a prothrombotic state, and are associated with inflammatory processes.11 Thus, an excess of circulating fatty acids (lipotoxicity) is one of the strongest links between obesity and the development of metabolic syndrome and/or cardiovascular disease. When calorie consumption exceeds energy expenditure, a metabolic state develops that promotes adipocyte hypertrophy (increased size) and hyperplasia (increased number).12 The latter involves mobilization of stem cells toward the adipocyte lineage (adipogenesis). New or small adipocytes are more sensitive to insulin and show a marked capacity for the uptake of free fatty acids and triglycerides in the postprandial period.4 As adipocytes increase in size (hypertrophy), they become dysfunctional, losing their ability to protect against systemic lipotoxicity, and ectopic fat begins to accumulate. These distended adipocytes become hyperlipolytic and resistant to insulin and its antilipolytic signals. Another important function performed by adipocytes is that of endocrines cells, as discussed below.
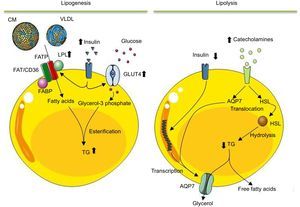
Diagram illustrating the lipogenesis and lipolysis processes occurring in mature adipocytes. After eating and an increase in blood insulin, lipogenesis is activated in adipocytes. In this process, adipocytes, via lipoprotein lipase, degrade the triglycerides of chylomicrons and of very-low-density lipoprotein to fatty acids. These molecules enter the adipocyte to be esterified with glycerol-3 phosphate and synthesize the triglycerides that are stored in the lipid vacuole. In the adipocyte, insulin not only stimulates lipoprotein lipase synthesis, but also stimulates uptake and metabolism of glucose to glycerol-3 phosphate. In contrast, during lipolysis, the triglycerides stored are mobilized to produce free fatty acids and glycerol to meet the energy requirements of the body. Catabolic hormones, secreted in response to a low blood concentration of glucose, activate the synthesis and movement of hormone-sensitive lipase from the cytosol to the surface of the lipid vacuole, where hormone-sensitive lipase hydrolyzes triglycerides. The fatty acids produced are secreted as free fatty acids to the circulation, where they will be transported by albumin to the target organs to be oxidized to produce energy. Similarly, the glycerol derived from lipolysis is also released into the circulation to be used by the liver as a source of carbon. AQP7, aquaporin-7; CM, chylomicrons; FABP, fatty acid-binding protein; FAT/CD36, fatty acid translocase; FATP, fatty acid transport protein; GLUT4, glucose transporter type 4; HSL, hormone-sensitive lipase; LPL, lipoprotein lipase; TG, triglycerides; VLDL, very-low-density lipoprotein.
The other component of WAT is the SVF. Although the cells constituting the SVF remain to be fully defined, they include adipocyte precursors and vascular and blood cells.13 Pericytes form the vasculature of adipose tissue, together with endothelial and smooth muscle cells. The extent and characteristics of this capillary network are crucial for processes such as the growth, function, and development of adipose tissue.14 Adipocytes and other cells of the SVF secrete proangiogenic factors, ensuring that the tissue has a generous blood supply. In addition, adipose tissue, via its resident immune system cells, strongly controls the metabolism of the body. In nonobese individuals, these cells are involved in elimination of necrotic adipocytes, remodeling of the extracellular matrix, angiogenesis, adipogenesis, and maintaining insulin sensitivity. However, in obese individuals, the number of immune system cells increases; these cells acquire a proinflammatory phenotype and release a huge number of cytokines in charge of recruiting and activating other immune system cells and inducing insulin resistance syndrome in adipose tissue.15 Macrophages are the cells with the most important role in the acquisition of the low-grade chronic proinflammatory state that characterizes obesity. During adipose tissue expansion, there is a greater recruitment of M1 macrophages (a proinflammatory phenotype). These macrophages secrete most of the proinflammatory cytokines found in obese adipose tissue,16 whereas the resident M2 macrophages show an anti-inflammatory phenotype.17 Finally, in the SVF, there are adipose-derived stem cells (ADSCs) and preadipocytes. These cell populations are in charge of maintaining adipocyte population renewal in physiological conditions and play a vital role in the obesity-related expansion of adipose tissue. The differences between these 2 cell groups are poorly defined. Both ADSCs and preadipocytes show a similar morphology. However, whereas ADSCs can differentiate into other lineages and show a large capacity for self-renewal, preadipocytes have lost these differentiation capabilities and can only generate mature adipocytes.18
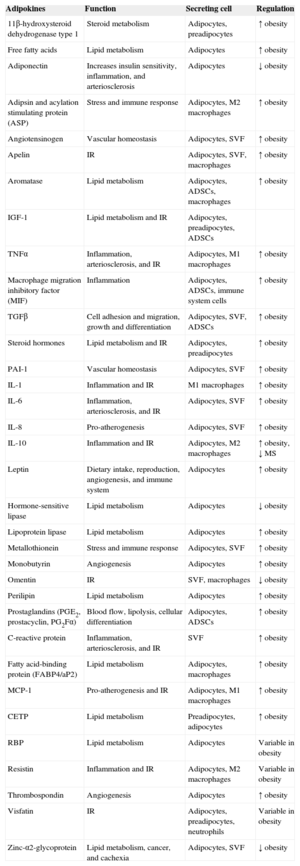
Factors Secreted by White Adipose TissueThe WAT secretes a multitude of bioactive peptides, known under the umbrella term of adipocytokines or adipokines (Table 1).19,20 However, many of these factors are not only secreted by adipocytes, but also by the cells constituting the SVF, such as macrophages and ADSCs. Through these secreted factors, adipose tissue participates in the autocrine and paracrine regulation of adipose tissue itself, as well as affecting the function of other organs. In addition, adipose tissue is in charge of regulating energy homeostasis and body weight, insulin sensitivity, and various functions of the immune, vascular, and reproductive systems.20 This endocrine function of adipose tissue explains the pathophysiological relationship between excess body fat and its associated pathological states, because obesity and/or metabolic syndrome trigger a dysregulation of the secreted amounts of these molecules.21
Factors Secreted by Adipose Tissue
| Adipokines | Function | Secreting cell | Regulation |
|---|---|---|---|
| 11β-hydroxysteroid dehydrogenase type 1 | Steroid metabolism | Adipocytes, preadipocytes | ↑ obesity |
| Free fatty acids | Lipid metabolism | Adipocytes | ↑ obesity |
| Adiponectin | Increases insulin sensitivity, inflammation, and arteriosclerosis | Adipocytes | ↓ obesity |
| Adipsin and acylation stimulating protein (ASP) | Stress and immune response | Adipocytes, M2 macrophages | ↑ obesity |
| Angiotensinogen | Vascular homeostasis | Adipocytes, SVF | ↑ obesity |
| Apelin | IR | Adipocytes, SVF, macrophages | ↑ obesity |
| Aromatase | Lipid metabolism | Adipocytes, ADSCs, macrophages | ↑ obesity |
| IGF-1 | Lipid metabolism and IR | Adipocytes, preadipocytes, ADSCs | |
| TNFα | Inflammation, arteriosclerosis, and IR | Adipocytes, M1 macrophages | ↑ obesity |
| Macrophage migration inhibitory factor (MIF) | Inflammation | Adipocytes, ADSCs, immune system cells | ↑ obesity |
| TGFβ | Cell adhesion and migration, growth and differentiation | Adipocytes, SVF, ADSCs | ↑ obesity |
| Steroid hormones | Lipid metabolism and IR | Adipocytes, preadipocytes | ↑ obesity |
| PAI-1 | Vascular homeostasis | Adipocytes, SVF | ↑ obesity |
| IL-1 | Inflammation and IR | M1 macrophages | ↑ obesity |
| IL-6 | Inflammation, arteriosclerosis, and IR | Adipocytes, SVF | ↑ obesity |
| IL-8 | Pro-atherogenesis | Adipocytes, SVF | ↑ obesity |
| IL-10 | Inflammation and IR | Adipocytes, M2 macrophages | ↑ obesity, ↓ MS |
| Leptin | Dietary intake, reproduction, angiogenesis, and immune system | Adipocytes | ↑ obesity |
| Hormone-sensitive lipase | Lipid metabolism | Adipocytes | ↓ obesity |
| Lipoprotein lipase | Lipid metabolism | Adipocytes | ↑ obesity |
| Metallothionein | Stress and immune response | Adipocytes, SVF | ↑ obesity |
| Monobutyrin | Angiogenesis | Adipocytes | ↑ obesity |
| Omentin | IR | SVF, macrophages | ↓ obesity |
| Perilipin | Lipid metabolism | Adipocytes | ↑ obesity |
| Prostaglandins (PGE2, prostacyclin, PG2Fα) | Blood flow, lipolysis, cellular differentiation | Adipocytes, ADSCs | ↑ obesity |
| C-reactive protein | Inflammation, arteriosclerosis, and IR | SVF | ↑ obesity |
| Fatty acid-binding protein (FABP4/aP2) | Lipid metabolism | Adipocytes, macrophages | ↑ obesity |
| MCP-1 | Pro-atherogenesis and IR | Adipocytes, M1 macrophages | ↑ obesity |
| CETP | Lipid metabolism | Preadipocytes, adipocytes | ↑ obesity |
| RBP | Lipid metabolism | Adipocytes | Variable in obesity |
| Resistin | Inflammation and IR | Adipocytes, M2 macrophages | Variable in obesity |
| Thrombospondin | Angiogenesis | Adipocytes | ↑ obesity |
| Visfatin | IR | Adipocytes, preadipocytes, neutrophils | Variable in obesity |
| Zinc-α2-glycoprotein | Lipid metabolism, cancer, and cachexia | Adipocytes, SVF | ↓ obesity |
ADSCs, adipose-derived stem cells; CETP, cholesteryl ester transfer protein; IGF-1, insulin-like growth factor 1; IR, insulin resistance; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; MS, metabolic syndrome; RBP, retinol-binding protein; PAI-I, plasminogen activator inhibitor-1; SVF, stromal vascular fraction; TGFβ, transforming growth factor beta; TNFα, tumor necrosis factor alpha.
Adapted from Ronti et al.20
For many years, the hyperplastic growth of adipose tissue was believed to be due to the existence of a unipotent progenitor cell population, the preadipocytes. However, in 2001, Zuk et al22 identified the existence of MSCs with self-renewal and multipotent capacities in adipose tissue. Since then, adipose tissue has been considered a source of MSCs for use in cell therapy.22
Origin of Adipose-derived Stem CellsSince adipocytes and their progenitors were found to originate from MSCs,23 it has been noted that ADSCs could be derived from mesenchymal lineage cells from the bone marrow. Indeed, the cells of the SVF show various similarities with those of the bone marrow. Both stromae contain a heterogeneous population of MSCs with the ability to differentiate into various lineages (adipocytic, chondrocytic, and myogenic) according to culture conditions.24 Mansilla et al25 noted that the bone marrow is the principal producer of the MSCs that supply the populations of MSCs found in other peripheral organs (peripheral reservoirs). Additionally, these authors showed that the cells are maintained in a quiescent and undifferentiated state until they are “called” to proliferate and move to the required tissues. Indeed, although there are practically no MSCs in the circulation of healthy individuals, these stem cells are mobilized toward damaged regions to participate in tissue repair and regeneration.26 Thus, it can be inferred that obese adipose tissue, as an important source of chemotactic factors, would act as a niche where circulating MSCs could home to and differentiate into adipocytes.25
Characteristics of Adipose-derived Stem CellsAdipose-derived stem cells show the typical characteristics of MSCs proposed by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy:27
- •
They must adhere to plastic while maintained in standard culture conditions.
- •
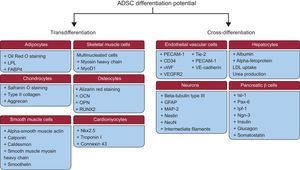
They must be able to differentiate into osteogenic, adipogenic, and chondrogenic lineages (Figure 2).
Figure 2.Adipose-derived stem cells differentiation potential. Adipose-derived stem cells are able to differentiate to other types of cells of the same mesodermal lineage (transdifferentiation) or to other types of cells from another lineage (cross-differentiation). ADSCs, adipose-derived stem cells; FABP4, fatty acid-binding protein 4; GFAP, glial fibrillary acidic protein; Ipf-1, insulin promoter factor 1; Isl-1, islet 1; LDL, low-density lipoprotein; LPL, lipoprotein lipase; MAP-2, microtubule-associated protein 2; MyoD1, myogenic differentiation factor 1; NeuN, neuronal nuclear antigen; Ngn-3, neurogenin 3; Nkx2.5, NK2 homeobox 5; OCN, osteocalcin; OPN, osteopontin; Pax-6, paired box protein 6; PECAM-1, platelet endothelial cell adhesion molecule 1; RUNX2, runt-related transcription factor 2; Tie-2, angiopoietin receptor 2; VE, vascular endothelial; VEGFR2, vascular endothelial growth factor 2; vWF, von Willebrand factor.
(0.37MB). - •
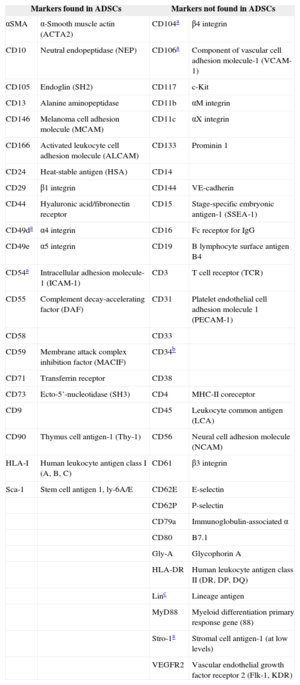
They must express the surface markers CD105, CD73, and CD90 and not express CD45, CD34, CD14 or CD11b, CD79a, or CD19, or HLA-II surface molecules.
Although ADSCs do not express a single surface marker that enables their identification, they express the characteristic markers of MSCs in conjunction with some markers expressed by nonprogenitor lines (Table 2).
Characteristic Cell Surface Markers of Adipose-derived Stem Cells
| Markers found in ADSCs | Markers not found in ADSCs | ||
|---|---|---|---|
| αSMA | α-Smooth muscle actin (ACTA2) | CD104a | β4 integrin |
| CD10 | Neutral endopeptidase (NEP) | CD106a | Component of vascular cell adhesion molecule-1 (VCAM-1) |
| CD105 | Endoglin (SH2) | CD117 | c-Kit |
| CD13 | Alanine aminopeptidase | CD11b | αM integrin |
| CD146 | Melanoma cell adhesion molecule (MCAM) | CD11c | αX integrin |
| CD166 | Activated leukocyte cell adhesion molecule (ALCAM) | CD133 | Prominin 1 |
| CD24 | Heat-stable antigen (HSA) | CD14 | |
| CD29 | β1 integrin | CD144 | VE-cadherin |
| CD44 | Hyaluronic acid/fibronectin receptor | CD15 | Stage-specific embryonic antigen-1 (SSEA-1) |
| CD49da | α4 integrin | CD16 | Fc receptor for IgG |
| CD49e | α5 integrin | CD19 | B lymphocyte surface antigen B4 |
| CD54a | Intracellular adhesion molecule-1 (ICAM-1) | CD3 | T cell receptor (TCR) |
| CD55 | Complement decay-accelerating factor (DAF) | CD31 | Platelet endothelial cell adhesion molecule 1 (PECAM-1) |
| CD58 | CD33 | ||
| CD59 | Membrane attack complex inhibition factor (MACIF) | CD34b | |
| CD71 | Transferrin receptor | CD38 | |
| CD73 | Ecto-5’-nucleotidase (SH3) | CD4 | MHC-II coreceptor |
| CD9 | CD45 | Leukocyte common antigen (LCA) | |
| CD90 | Thymus cell antigen-1 (Thy-1) | CD56 | Neural cell adhesion molecule (NCAM) |
| HLA-I | Human leukocyte antigen class I (A, B, C) | CD61 | β3 integrin |
| Sca-1 | Stem cell antigen 1, ly-6A/E | CD62E | E-selectin |
| CD62P | P-selectin | ||
| CD79a | Immunoglobulin-associated α | ||
| CD80 | B7.1 | ||
| Gly-A | Glycophorin A | ||
| HLA-DR | Human leukocyte antigen class II (DR, DP, DQ) | ||
| Linc | Lineage antigen | ||
| MyD88 | Myeloid differentiation primary response gene (88) | ||
| Stro-1a | Stromal cell antigen-1 (at low levels) | ||
| VEGFR2 | Vascular endothelial growth factor receptor 2 (Flk-1, KDR) | ||
ADSCs, adipose-derived stem cells; BM-MSCs, bone marrow-derived mesenchymal stem cells.
As metabolically active cells, ADSCs play important roles in the revascularization of damaged tissue, apoptosis inhibition, and immunomodulation. These stem cells secrete a large quantity of extracellular matrix factors and a large number of cytokines and growth, angiogenic, and antiapoptotic factors.28 Indeed, a large part of the beneficial effects of cell therapy with ADSCs is believed to be due to their robust secretion of paracrine factors. Importantly, these angiogenic and antiapoptotic factors are secreted in bioactive quantities, and this secretion is increased under hypoxia.29
Source-related Differences in Adipose-derived Stem CellsThe metabolic differences between subcutaneous and visceral adipose tissue may be due to the intrinsic characteristics of the cells resident in each tissue, including ADSCs. Indeed, adipocytes differentiated in vitro from ADSCs derived from the 2 sources show differences inherent to the source tissues.30 These differences are stable and are maintained after the ADSCs have been isolated and cultured in vitro.31 Various studies have reported differences in the proliferation, differentiation, and apoptotic potentials, as well as gene expression patterns, of ADSCs from different adipose tissues.32–34 Adipose-derived stem cells from subcutaneous adipose tissue show greater adipogenic differentiation capacity than ADSCs from visceral adipose tissue.32 The low capacity for differentiation of the visceral ADSCs could partly explain why fat accumulates in already existing adipocytes and, consequently, why the size of their lipid vacuoles increases. In contrast, the greater differentiation capacity of subcutaneous ADSCs would result in lipid accumulation in new adipocytes with smaller vacuoles.35 Accordingly, the size of the lipid vacuoles of visceral adipocytes correlates with the concentrations of circulating lipids, whereas the degree of hyperplasia and size of the subcutaneous adipocytes are more related to the plasma concentrations of glucose and insulin and to insulin sensitivity.36 However, it is still unknown how the ADSCs of each adipose tissue acquire their characteristic phenotypes and at what developmental stage. The regional characteristics of the different ADSCs might be regulated epigenetically, appearing during early developmental phases and being established later by the environment of each adipose tissue and of each individual. Knowledge of the differences between the ADSCs of the different adipose tissues would be of great interest for a better understanding of the biology of the tissue and the development of its different deposits.
Effect of Cardiovascular Risk Factors on Adipose-derived Stem CellsVarious studies have shown that hypercholesterolemia, types 1 and 2 diabetes mellitus, hypertension, and smoking negatively affect endogenous stem/progenitor cells. Recently, our group has reported that type 2 diabetes mellitus negatively affects the pluripotency and self-renewal capacities of ADSCs, altering the main pathways involved in the maintenance of stem cells and their differentiation and angiogenic potentials.37
Obesity has also been described as a disease that affects ADSCs. Van Harmelen et al38 found that the adipogenic differentiation capacity of ADSCs of subcutaneous mammary adipose tissue is decreased in women with a high body mass index. Subsequently, Nair et al39 reported that ADSCs from subcutaneous adipose tissue of obese Pima Indian individuals had higher expression of proinflammatory genes than those of nonobese individuals. Recently, it has been reported that morbidly obese individuals have ADSCs with impaired proliferation, differentiation, and angiogenic capacities, which negatively affect the regenerative capacity of these cells.40 Additionally, the ADSCs of obese patients show lower levels of multipotency markers, increased commitment toward an adipocyte lineage, and higher expression of proinflammatory genes than ADSCs derived from nonobese patients.41 In addition, the effect of obesity on ADSCs was seen in both those cells derived from subcutaneous adipose tissue and those derived from visceral tissue.42
ADIPOSE-DERIVED STEM CELLS IN CELL THERAPYBone marrow-derived MSCs have been used for many years as the main source of stem cells for regenerative medicine and as an alternative to embryonic stem cells.43 However, due to the ease of acquisition and isolation of ADSCs and the large quantity obtained, they have become an important alternative source of stem cells with considerable advantages over bone marrow-derived MSCs.44,45 Initially, the reparative/regenerative capacity of ADSCs was proposed to be due to their ability to differentiate into other cell types.46 However, studies performed in recent years have confirmed that the reparative potential of ADSCs is largely due to their release of paracrine factors.44,45
Adipose-derived Stem Cells in Ischemic Heart DiseaseExperimental StudiesIn recent years, numerous preclinical studies and some clinical studies have analyzed the safety, behavior, and efficacy of ADSCs in the treatment of ischemic injury, especially that of cardiac origin.47–51 The first study was performed in a rat model of heart cryoinjury and involved the injection of recently isolated ADSCs into the left ventricular cavity to simulate intracoronary administration.51 That study was the first to show that ADSCs home to the myocardium and express specific markers of cardiac cells. Similarly, functional and pathological analyses of the ADSC-treated animals revealed significantly improved global cardiac function and increased capillary density in the injury border zone compared with controls.51 Since then, the capacity of ADSCs to generate cardiomyocytes and vascular cells has become a topic of great experimental interest, as shown in Table 351-91 (studies performed in rodents and rabbits) and Table 492–101 (studies performed in porcine models). Notably, controversy surrounds the efficacy of the ADSCs. Whereas some studies have found engrafted ADSCs expressing specific cardiac markers (troponin I and myosin light chain),51,102–105 von Willebrand factor, and/or smooth muscle actin, other studies failed to discern the differentiation capacity of ADSCs (Tables 3 and 4).52,92 These differences in the in vivo differentiation potential of ADSCs could be due to differences in ADSC sources, procurement processes or culture media, animal models, or means of administration, or be due to the limits of histological analysis. Various groups have similarly found a low differentiation capacity of ADSCs in studies in vivo.52,53,92 All of these observations have led scientists to question whether the benefits derived from ADSC administration are directly related to the differentiation processes or if, in contrast, they are conditioned by ADSC secretion of paracrine factors.106–108
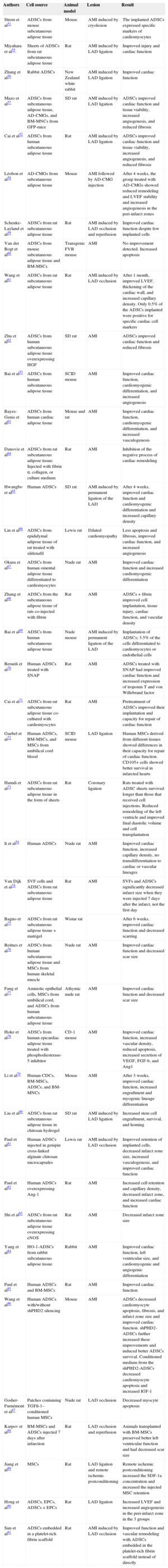
Experimental Studies Using Rodent and Rabbit Animal Models
| Authors | Cell source | Animal model | Lesion | Result |
|---|---|---|---|---|
| Strem et al51 | ADSCs from mouse subcutaneous adipose tissue | Mouse | AMI induced by cryolesion | The implanted ADSCs expressed specific markers of cardiomyocytes |
| Miyahara et al55 | Sheets of ADSCs from rat subcutaneous adipose tissue | Rat | AMI induced by LAD ligation | Improved injury and cardiac function |
| Zhang et al56 | Rabbit ADSCs | New Zealand white rabbit | AMI induced by LAD ligation | Improved cardiac function |
| Mazo et al57 | ADSCs from subcutaneous adipose tissue, AD-CMGs, and BM-MNCs from GFP-mice | SD rat | AMI induced by LAD ligation | ADSCs improved cardiac function and tissue viability, increased angiogenesis, and reduced fibrosis |
| Cai et al52 | ADSCs from human subcutaneous adipose tissue | Rat | AMI induced by LAD ligation | ADSCs improved cardiac function and tissue viability, increased angiogenesis, and reduced fibrosis |
| Léobon et al58 | AD-CMGs from subcutaneous adipose tissue | Mouse | AMI followed by AD-CMG injection | After 4 weeks, the group treated with AD-CMGs showed reduced remodeling and LVEF stability and increased angiogenesis in the peri-infarct zones |
| Schenke-Layland et al59 | ADSCs from rat subcutaneous adipose tissue | Rat | AMI induced by LAD occlusion and reperfusion | Improved cardiac function despite few implanted cells |
| Van der Bogt et al60 | ADSCs from mouse subcutaneous adipose tissue and BM-MSCs | Transgenic FVB mouse | AMI | No improvement detected. Increased apoptosis |
| Wang et al61 | ADSCs from rat subcutaneous adipose tissue | Rat | AMI induced by LAD occlusion | After 1 month, improved LVEF, thickening of the cardiac wall, and increased capillary density. Only 0.5% of the ADSCs implanted were positive for specific cardiac cell markers |
| Zhu et al62 | ADSCs from human subcutaneous adipose tissue overexpressing HGF | SD rat | AMI | ADSCs improved cardiac function and reduced fibrosis |
| Bai et al53 | ADSCs from human subcutaneous adipose tissue | SCID mouse | AMI | Improved cardiac function, cardiomyogenic differentiation, and increased angiogenesis |
| Bayes-Genis et al63 | ADSCs from human cardiac adipose tissue | Mouse and rat | AMI | Improved cardiac function, cardiomyogenic differentiation, and increased vasculogenesis |
| Danoviz et al64 | ADSCs from rat subcutaneous adipose tissue. Injected with fibrin α, collagen, or culture medium | Rat | AMI | Inhibition of the negative process of cardiac remodeling |
| Hwangbo et al65 | Human ADSCs | SD rat | AMI induced by permanent ligation of the LAD | After 4 weeks, improved cardiac function and cardiomyogenic differentiation and increased capillary density |
| Lin et al66 | ADSCs from epididymal adipose tissue of rat treated with sildenafil | Lewis rat | Dilated cardiomyopathy | Less apoptosis and fibrosis, improved cardiac function, and increased angiogenesis |
| Okura et al67 | ADSCs from human omental adipose tissue differentiated to cardiomyocytes | Nude rat | AMI | Improved cardiac function and increased cardiomyogenic differentiation |
| Zhang et al68 | ADSCs from the subcutaneous adipose tissue of rats co-injected with fibrin | Rat | AMI | ADSCs + fibrin improved cell implantation, tissue injury, cardiac function, and vascular density |
| Bai et al69 | ADSCs from human subcutaneous adipose tissue | Nude mouse | AMI induced by permanent ligation of the LAD | Implantation of ADSCs; 3.5% of the cells differentiated to cardiomyocytes or endothelial cells |
| Berardi et al70 | Human ADSCs treated with SNAP | Rat | AMI | ADSCs treated with SNAP had improved cardiac function and increased expression of troponin T and von Willebrand factor |
| Cai et al71 | ADSCs from rat subcutaneous adipose tissue co-cultured with cardiomyocytes | Rat | AMI | Pretreatment of ADSCs improved their implantation and capacity for repair of cardiac function |
| Gaebel et al72 | Human ADSCs, BM-MSCs, and MSCs from umbilical cord blood | SCID mouse | LAD ligation | Human MSCs derived from different tissues showed differences in their capacity for repair of cardiac function. CD105+ cells showed better survival in infarcted hearts |
| Hamdi et al73 | ADSCs from rat subcutaneous adipose tissue in the form of sheets | Rat | Coronary ligation | Rats treated with ADSC sheets survived longer than those that received cell injections. Reduced remodeling of the left ventricle and improved final diastolic volume and cell transplantation |
| Ii et al54 | Human ADSCs | Nude rat | AMI | Improved cardiac function, increased capillary density, no transdifferentiation to cardiac or vascular lineages |
| Van Dijk et al74 | SVF cells and ADSCs from rat subcutaneous adipose tissue | Rat | AMI | SVFs and ADSCs significantly decreased infarct size when they were injected 7 days after the infarct, not the first day |
| Bagno et al75 | ADSCs from rat subcutaneous adipose tissue + matrigel | Wistar rat | After 6 weeks, improved cardiac function and decreased scarring | |
| Beitnes et al76 | ADSCs from human subcutaneous adipose tissue and MSCs from human skeletal muscle | Nude rat | AMI | Improved cardiac function and decreased scar size |
| Fang et al77 | Amniotic epithelial cells, MSCs from umbilical cord, and ADSCs from human subcutaneous adipose tissue | Athymic nude rat | AMI | Improved cardiac function and decreased scar size |
| Hoke et al78 | ADSCs from human epicardiac adipose tissue treated with phosphodiesterase-5 inhibitor | CD-1 mouse | AMI | Improved cardiac function, increased vascular density, reduced apoptosis, increased secretion of VEGF, FGF-b, and Ang1 |
| Li et al79 | Human CDCs, BM-MSCs, ADSCs, and BM-MNCs | Mouse | AMI | After 3 weeks, improved cardiac function, increased engraftment and myogenic lineage differentiation |
| Liu et al80 | ADSCs from rat subcutaneous adipose tissue in chitosan hydrogel | SD rat | AMI induced by LAD ligation | Increased stem cell engraftment, survival, and homing |
| Paul et al81 | Human ADSCs injected in genipin cross-linked alginate chitosan microcapsules | Lewis rat | AMI induced by LAD occlusion | Improved retention of implanted cells, decreased infarct zone size, increased vasculogenesis, and improved cardiac function |
| Paul et al82 | Human ADSCs overexpressing Ang-1 | Rat | AMI | Increased cell retention and capillary density, decreased infarct zone, and increased cardiac function |
| Shi et al83 | ADSCs from rat subcutaneous adipose tissue overexpressing eNOS | Rat | AMI | Decreased infarct zone size |
| Yang et al84 | HO-1-ADSCs from rabbit subcutaneous adipose tissue | Rabbit | AMI | Improved cardiac function, left ventricular size, and cardiomyogenic and angiogenic differentiation |
| Paul et al85 | Human ADSCs and BM-MSCs | Rat | AMI | Improved cardiac function |
| Wang et al86 | Human ADSCs with/without shPHD2 silencing | Mouse | AMI | ADSCs decreased cardiomyocyte apoptosis, fibrosis, and infarct zone size and improved cardiac function. shPHD2-ADSCs further increased these improvements and induced better ADSCs survival. Conditioned medium from the shPHD2-ADSCs decreased cardiomyocyte apoptosis and increased IGF-1 |
| Godier-Furnémont et al87 | Patches containing TGFß-1–conditioned human MSCs | Nude rat | LAD occlusion | Decreased myocyte apoptosis |
| Karpov et al88 | BM-MSCs and ADSCs injected 7 days after infarction | Rat | LAD occlusion and reperfusion | Animals transplanted with BM-MSCs preserved better left ventricular function and had decreased scar size |
| Jiang et al89 | MSCs | Rat | LAD ligation and remote ischemic postconditioning | Remote ischemic postconditioning increased the SDF-1a concentration and increased the injected MSC retention |
| Hong et al90 | ADSCs, EPCs, ADSCs + EPCs | Rat | LAD ligation | Increased LVEF and increased angiogenesis in the peri-infarct zone in the 3 groups |
| Sun et al91 | ADSCs embedded in a platelet-rich fibrin scaffold | Rat | AMI induced by LAD occlusion | Improved function and vascular remodeling with ADSCs embedded in the platelet-rich fibrin scaffold instead of directly |
AD-CMGs, ADSC-derived cardiomyogenic cells; ADSCs, adipose-derived stem cells; AMI, acute myocardial infarction; Ang-1, angiopoietin-1; b-FGF, basic fibroblast growth factor; BM-MNCs, bone marrow-derived mononuclear cells; BM-MSCs, bone marrow-derived mesenchymal stem cells; CDCs, cardiosphere-derived cells; eNOS, endothelial nitric oxide synthase; EPCs, endothelial progenitor cells; GFP, green fluorescent protein; HGF, hepatic growth factor; HO-1-ADSCs, adipose-derived stem cells transduced with heme oxygenase-1; IGF-1, insulin-like growth factor 1; LAD, left anterior descending coronary artery; LVEF, left ventricular ejection fraction; MSCs, mesenchymal stem cells; SCID, severe combined immunodeficiency; SD, Sprague Dawley; shPHD2, prolyl hydroxylase domain protein 2; SNAP, S-Nitroso-N-acetyl-DL-penicillamine; SVF, stromal vascular fraction; TGFβ-1, transforming growth factor β1; VEGF, vascular endothelial growth factor.
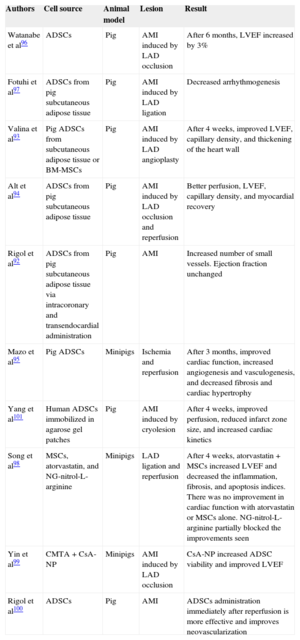
Preclinical Studies with Porcine Models
| Authors | Cell source | Animal model | Lesion | Result |
|---|---|---|---|---|
| Watanabe et al96 | ADSCs | Pig | AMI induced by LAD occlusion | After 6 months, LVEF increased by 3% |
| Fotuhi et al97 | ADSCs from pig subcutaneous adipose tissue | Pig | AMI induced by LAD ligation | Decreased arrhythmogenesis |
| Valina et al93 | Pig ADSCs from subcutaneous adipose tissue or BM-MSCs | Pig | AMI induced by LAD angioplasty | After 4 weeks, improved LVEF, capillary density, and thickening of the heart wall |
| Alt et al94 | ADSCs from pig subcutaneous adipose tissue | Pig | AMI induced by LAD occlusion and reperfusion | Better perfusion, LVEF, capillary density, and myocardial recovery |
| Rigol et al92 | ADSCs from pig subcutaneous adipose tissue via intracoronary and transendocardial administration | Pig | AMI | Increased number of small vessels. Ejection fraction unchanged |
| Mazo et al95 | Pig ADSCs | Minipigs | Ischemia and reperfusion | After 3 months, improved cardiac function, increased angiogenesis and vasculogenesis, and decreased fibrosis and cardiac hypertrophy |
| Yang et al101 | Human ADSCs immobilized in agarose gel patches | Pig | AMI induced by cryolesion | After 4 weeks, improved perfusion, reduced infarct zone size, and increased cardiac kinetics |
| Song et al98 | MSCs, atorvastatin, and NG-nitrol-L-arginine | Minipigs | LAD ligation and reperfusion | After 4 weeks, atorvastatin + MSCs increased LVEF and decreased the inflammation, fibrosis, and apoptosis indices. There was no improvement in cardiac function with atorvastatin or MSCs alone. NG-nitrol-L-arginine partially blocked the improvements seen |
| Yin et al99 | CMTA + CsA-NP | Minipigs | AMI induced by LAD occlusion | CsA-NP increased ADSC viability and improved LVEF |
| Rigol et al100 | ADSCs | Pig | AMI | ADSCs administration immediately after reperfusion is more effective and improves neovascularization |
ADSCs, adipose-derived stem cells; AMI, acute myocardial infarction; BM-MSCs, bone marrow-derived mesenchymal stem cells; CsA-NP, cyclosporine A-nanoparticle emulsion; LAD, left anterior descending coronary artery; LVEF, left ventricular ejection fraction; MSCs, mesenchymal stem cells.
Another important function of ADSCs related to ischemic heart disease is derived from their angiogenic potential.52,109 Because ADSCs secrete a large number of proangiogenic cytokines and cytoprotective factors, they are an ideal cellular source for angiogenic therapy and apoptosis inhibition.29,110–112 An in vivo study showed that intramyocardial injection of human ADSCs significantly promoted angiogenesis and inhibited cell apoptosis in an infarcted heart 4 weeks after their injection.54 Moreover, ADSCs showed an increased expression of vascular endothelial and fibroblast growth factors and stromal cell-derived factor 1.54 Indeed, the interaction of stromal cell-derived factor 1 with its receptor induces the rapid mobilization of stem/progenitor cells from the bone marrow,113 which is essential for the revascularization of body systems.114 All of these results indicate that injected ADSCs cooperate with stem/progenitor cells of the bone marrow via cellular mobilization, which is promoted by stromal cell-derived factor 1 and which boosts angiogenesis and vasculogenesis in myocardial ischemia.54
The functional response of ADSCs can also be affected by oxygen concentration.29,115 Rehman et al29 found that ADSCs secreted up to 5 times more vascular endothelial growth factors if they were cultured under hypoxic conditions. Additionally, the conditioned supernatant of ADSCs cultured under hypoxic conditions increased the growth of endothelial cells and reduced their apoptosis. Recently, a study reported that hypoxic preconditioning of ADSCs increased their survival and paracrine effects in a hypoxia-inducible factor 1-mediated manner.116 Indeed, our laboratory group has shown that ADSCs cultured under hypoxic conditions had greatly improved proliferative capacity.40 These results showed that ADSCs respond to ischemic situations and promote angiogenesis via the secretion of vascular endothelial growth factors. As already mentioned, various experimental studies have shown that ADSCs are safe and efficacious (Tables 3 and 4). As can be seen in Table 4, 2 studies showed an increased capillary density in the area surrounding the infarcted heart and an increased cardiac function 1 month after a myocardial infarction when animals were treated with ADSCs, results similar to those observed after bone marrow-derived MSCs administration.93,94 A long-term follow-up study showed that, despite an inability to detect ADSCs in the myocardium 3 months after their injection, ADSC transplantation was associated with increased cardiac function, positive remodeling, and increased angiogenesis and vasculogenesis, confirming the long-term paracrine effect of these cells.95 However, these observations indicate that, despite all of the advantages of ADSCs, their low capacity for homing to ischemic tissue is an obstacle to their clinical use.117 Accordingly, various strategies are being explored to resolve the problem of ADSC survival and engraftment in host tissue, such as the administration of ADSCs together with a combination of growth factors,118 injection of genetically modified ADSCs,119 and/or the use of grafts/biomaterial scaffolds.120,121 Finally, it is necessary to mention that various studies have indicated that ADSCs, both in vitro and in vivo, can boost the differentiation of the vasculature into pericytes, cells capable of creating microvessels, preventing vascular regression, and promoting long-term microvessel maintenance.122,123
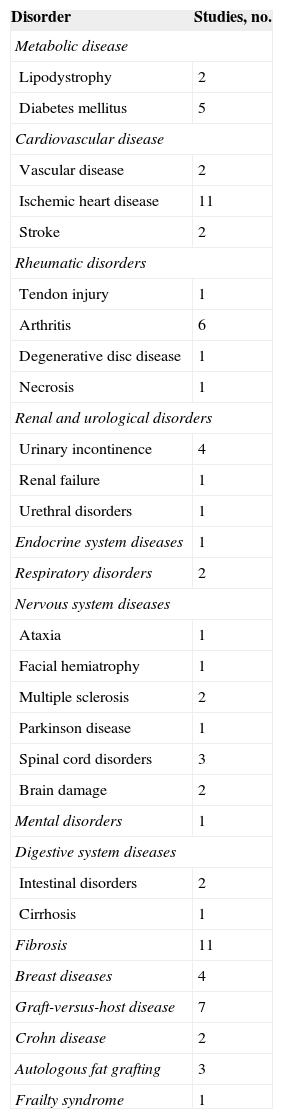
Clinical TrialsThe evidence found in the experimental studies of the potential of ADSCs to repair the ischemic myocardium and restore its functional capacity has prompted the performance of clinical trials in this area. However, although some researchers believe that ADSCs will be used in the coming years as a cell therapy aimed at repairing the damaged heart, others believe that there are many unknowns to be resolved before these cells can be clinically used. So far, ADSCs have been satisfactorily used to treat some diseases, such as Crohn's fistula, osteogenesis imperfecta, and breast reconstruction after a partial mastectomy (Table 5). However, the use of ADSCs in the field of ischemic heart disease is still in phase I-II. Various clinical trials have been initiated to determine the feasibility, safety, and efficacy of the use of ADSCs in patients who have had an acute myocardial infarction (APOLLO, ADI-ME-CHF-002, ADVANCE, and ACUTE MI), have chronic ischemic heart disease (PRECISE, MyStromalCell, ATHENA, and ATHENA II), or nonischemic cardiomyopathy (ADI-ME-CHF-002). Of these, the APOLLO124, PRECISE,125 and MyStromalCell126 trials have been completed. These studies showed that the use of ADSCs is safe and feasible. In addition, the results indicated that ADSC use preserves cardiac function, improves cardiac perfusion, and even reduces scar tissue size, thereby reinforcing the findings of the previous preclinical trials.
Clinical Trials Performed with Adipose-derived Stem Cells
| Disorder | Studies, no. |
|---|---|
| Metabolic disease | |
| Lipodystrophy | 2 |
| Diabetes mellitus | 5 |
| Cardiovascular disease | |
| Vascular disease | 2 |
| Ischemic heart disease | 11 |
| Stroke | 2 |
| Rheumatic disorders | |
| Tendon injury | 1 |
| Arthritis | 6 |
| Degenerative disc disease | 1 |
| Necrosis | 1 |
| Renal and urological disorders | |
| Urinary incontinence | 4 |
| Renal failure | 1 |
| Urethral disorders | 1 |
| Endocrine system diseases | 1 |
| Respiratory disorders | 2 |
| Nervous system diseases | |
| Ataxia | 1 |
| Facial hemiatrophy | 1 |
| Multiple sclerosis | 2 |
| Parkinson disease | 1 |
| Spinal cord disorders | 3 |
| Brain damage | 2 |
| Mental disorders | 1 |
| Digestive system diseases | |
| Intestinal disorders | 2 |
| Cirrhosis | 1 |
| Fibrosis | 11 |
| Breast diseases | 4 |
| Graft-versus-host disease | 7 |
| Crohn disease | 2 |
| Autologous fat grafting | 3 |
| Frailty syndrome | 1 |
Thus, ADSCs are appearing as a viable cell therapy alternative in various medical fields, which will require a better understanding of the mechanisms used by these cells or their paracrine factors in tissue regeneration/recovery, as well as the key molecular factors promoting the differentiation of ADSCs to different lineages. Additionally, it remains to be determined whether therapeutic efficacy is affected by the anatomical source of the cells, the sex and age of the donor, or the presence of comorbidities. The possibility of using both autologous and allogeneic ADSCs should also be considered, because various independent studies have reported that ADSCs have a low immunostimulatory potential.127,128
FUNDINGPart of the work contained in this manuscript was financed by the Programa Nacional de Salud (SAF 2013-42962-R, awarded to L. Badimon; SAF 2012-40208 awarded to G. Vilahur), the Instituto de Salud Carlos III (TerCel [Red de Terapia Celular] RD12/0019/0026), and the Fundación Jesús Serra (FIC-Barcelona).
CONFLICTS OF INTERESTNone declared.
We thank the Fundación Jesús Serra of Barcelona for its continuing support. G. Vilahur is a Ramón y Cajal researcher with a contract with the Secretaría de Estado de Investigación, Desarrollo e Innovación from the Ministerio de Economía y Competitividad of Spain (RyC-2009-5495).