To assess the prevalence of atherogenic dyslipidemia in hypertensive patients and its relationship with risk profile and blood pressure control.
MethodsThe study included 24 351 hypertensive patients from the Spanish Ambulatory Blood Pressure Monitoring Registry. Atherogenic dyslipidemia was defined as the presence of hypertriglyceridemia (> 150mg/dL) and low levels of high-density lipoprotein cholesterol (< 40mg/dL in men and < 46mg/dL in women). Blood pressure control was assessed by office and ambulatory monitoring.
ResultsAtherogenic dyslipidemia was present in 2705 patients (11.1%). Of these, 30% had hypertriglyceridemia and 21.7% had low levels of high-density lipoprotein cholesterol. Compared with patients without these risk factors, the former group were more often male (60% vs 52%), younger (57 years vs 59 years), had other risk factors and organ damage (microalbuminuria, reduced estimated glomerular filtration rate, and left ventricular hypertrophy), worse office, diurnal, and nocturnal blood pressure values (odds ratio 1.09, 1.06, and 1.10, respectively), and the lowest nocturnal blood pressure reduction (odds ratio=1.07), despite the greater use of antihypertensive drugs.
ConclusionsAtherogenic dyslipidemia is present in more than 10% of hypertensive patients and is associated with other risk factors, organ damage, and poorer blood pressure control. Greater therapeutic effort is needed to reduce overall risk in these patients.
Keywords
Hypertension is considered to be the main risk factor for death worldwide because of its impact on cardiovascular disease, which remains the leading cause of death.1However, the risk attributable to elevated blood pressure (BP) levels is modified by the presence of other risk factors. Lipid disorders are among the most important additional risk factors. The risk of hypertension and of dyslipidemia is exacerbated by the high prevalence of hypertension and dyslipidemia in combination.2,3These disorders are probably found together because they share common pathogenic factors, especially those stemming from the environment.4,5It is known that patients with hypertension have a higher rate of lipid disorders than the general population.6,7
The 2 main lipid disorders that appear in hypertensive patients are high levels of low-density lipoprotein cholesterol (LDL-C) and atherogenic dyslipidemia (AD), which is characterized by elevated triglyceride levels and low levels of high-density lipoprotein cholesterol (HDL-C) with or without high levels of LDL-C or non-HDL cholesterol. Non-HDL cholesterol is transported by all atherogenic lipoproteins and is used as a proxy for LDL-C levels when these cannot be calculated due to the presence of hypertriglyceridemia. Atherogenic dyslipidemia may occur in isolation or more often with other body fat distribution and carbohydrate metabolism abnormalities known as metabolic syndrome.8 Little is known about the impact of AD on BP control or about the effects of therapeutic efforts in patients with AD.
Thus, the aim of the study was to assess the prevalence of AD and its components (low HDL-C levels and increased triglyceride levels) and its relationship with office and ambulatory BP control and silent organ damage in a large cohort of hypertensive patients.
METHODSPatient SelectionThe study included 24 351 patients from the Spanish Ambulatory Blood Pressure Monitoring registry (ABPM). This registry is based on the distribution of BP monitors to more than 1000 physicians in the 17 Spanish autonomous communities. The physicians were mainly from primary care centers, but were also from specialized cardiology, nephrology, and internal medicine units. The ABPM records and clinical patient data were sent to an internet-based platform. The physicians then received a report in realtime describing the main results. The details of the process have been described in previous articles published by our group.9–13
The registry began in June 2004 and by December 31, 2010 the database included 104 904 patients. The present study included patients with satisfactory ABPM record quality and basic clinical data, and laboratory data concomitant with the APBM readings (3 months) that included total cholesterol, HDL-C and triglyceride levels, and the number and type of antihypertensive and lipid-lowering drugs. Of the 24 351 patients included, 54.6% were men and the mean age was 59 (SD, 14) years. In total, 16 254 were receiving some type of antihypertensive treatment and the remaining 8097 patients were receiving no treatment.
Blood Pressure Measurement and Definition of VariablesOffice systolic and diastolic BP were measured twice with a mercury sphygmomanometer or validated semiautomatic device after a 5-minute rest. The mean of the 2 measurements was used in the analysis.
Ambulatory blood pressure monitoring was preferentially conducted on a day of normal activity using a cuff matched to the size of the patient's arm. A record was considered valid when the percentage of successful readings was ≥ 70% of the total number of readings and the time between readings was never more than 1 hour. The means and standard deviations of 24-hour, diurnal (activity), and nocturnal (resting) systolic and diastolic BP and heart rate values were calculated based on patient logs.
Normotension/hypertension or control/no control (with or without antihypertensive treatment) were defined according to Spanish14 and international15 recommendations at the cutoff values of < 140/90mmHg for office BP, 130/80mmHg for 24-hour BP, 135/85mmHg for diurnal BP, and 120/70mmHg for nocturnal BP.
The circadian pattern was estimated by calculating the night/day ratio for each parameter (systolic BP, diastolic BP, and heart rate). Risk profiles were defined according to the decrease in nocturnal systolic BP compared with diurnal systolic BP. The following categories were established: dipper (10%-20% decrease), extreme dipper (more than 20% decrease), nondipper (less than 10% decrease), and riser (nocturnal systolic BP higher than diurnal systolic BP).11
Definition of Clinical and Laboratory VariablesBody mass index (BMI) was calculated by dividing weight (in kilos) by the square of height (in meters). Patients were considered to be smokers if they had consumed any kind of tobacco in the last year. Diabetes mellitus was defined as glycemia > 125mg/dL or treatment with antidiabetic agents. Left ventricular hypertrophy was defined according to electrocardiographic criteria (Sokolow-Lyon index > 38mm or Cornell voltage-duration product> 2440mm/ms). Microalbuminuria was defined as urinary albumin excretion > 30mg/g. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate < 60mL/min/1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration equation.16 Prior cardiovascular events were defined as documented myocardial infarction, stroke, hospitalization for unstable angina, or coronary or peripheral vascularization procedure.
Lipid levels (total cholesterol, triglycerides, HDL-C and LDL-C) were measured in the laboratories of each participating center using standard analytical procedures. Atherogenic dyslipidemia was defined as triglyceride levels > 150mg/dL and HDL-C levels < 40mg/dL in men and < 46mg/dL in women, regardless of lipid-lowering drug treatment and according to the normal values proposed for hypertensive patients.14,15
Statistical AnalysisData are expressed as mean (standard deviation) for continuous variables with a normal distribution, median [interquartile range] for continuous variables with a non-Gaussian distribution (Kolmogorov-Smirnoff), or as percentages for categorical variables. Four groups were established: Patients with normal triglyceride and HDL-C levels, patients with normal triglyceride and low HDL-C levels, patients with high triglyceride and normal HDL-C levels, and patients with AD. Depending on the normality of the distribution, groups were compared using chi-square, analysis of variance, or nonparametric Kruskal-Wallis tests. Bonferroni correction was applied for comparisons between pairs of groups. After adjusting for age, sex, and the number of antihypertensive drugs, odds ratios (OR) and 95% confidence intervals (95% CI) were calculated for the association between the abnormalities described and poor office, diurnal, and nocturnal BP control and the circadian profile(nondipper/riser). The statistical analysis was performed using the SPSS v 19 software package (IBM Corp., Amronk, New York, United States). A P value of < .05 was used as a cutoff for statistical significance.
RESULTSOf the 24 351 patients included, 16 254 (67%) were receiving antihypertensive treatment and 8097 (33%) were receiving no treatment. In total, 6774 patients were receiving lipid-lowering drugs, which mainly consisted of statins (24.9%), fibrates (2.2%), and ezetimibe (2.1%).
In total, 5273 patients (21.7%) had HDL-C levels < 40mg/dL (men) or < 46mg/dL (women) and 7316 (30.0%) patients had triglyceride levels > 150mg/dL. Atherogenic dyslipidemia was present in 2705 (11.1%) patients and was significantly higher (P < .001) in the group receiving antihypertensive treatment (12.3%) than in those without treatment (8.8%).
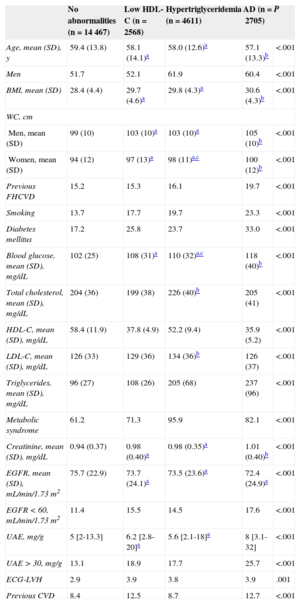
Table 1 shows the clinical and laboratory characteristics and prevalence of organ damage of the4 groups: no abnormalities (n=14 447), low HDL-C levels (n=2568), high triglyceride levels (n=4611), and AD (n=2705). Cardiovascular risk factors (general and abdominal obesity, family history of premature cardiovascular disease, smoking, and diabetes mellitus) were more frequent in the AD patients. Regarding organ damage, AD patients more frequently had a glomerular filtration rate < 60mL/min/1.73 m2, microalbuminuria, left ventricular hypertrophy, and a family history of cardiovascular disease. Patients with either hypertriglyceridemia or low HDL-C had an intermediate risk profile between patients with AD and those without abnormalities.
Office and Laboratory Parameters and Prevalence of Organ Damage in Patients Without Hypertriglyceridemia or Low LDL-C Levels, With Low HDL-C Levels, With Hypertriglyceridemia, or With Both Disorders (Atherogenic Dyslipidemia)
| No abnormalities (n=14 467) | Low HDL-C (n=2568) | Hypertriglyceridemia (n=4611) | AD (n=2705) | P | |
|---|---|---|---|---|---|
| Age, mean (SD), y | 59.4 (13.8) | 58.1 (14.1)a | 58.0 (12.6)a | 57.1 (13.3)b | <.001 |
| Men | 51.7 | 52.1 | 61.9 | 60.4 | <.001 |
| BMI, mean (SD) | 28.4 (4.4) | 29.7 (4.6)a | 29.8 (4.3)a | 30.6 (4.3)b | <.001 |
| WC, cm | |||||
| Men, mean (SD) | 99 (10) | 103 (10)a | 103 (10)a | 105 (10)b | <.001 |
| Women, mean (SD) | 94 (12) | 97 (13)a | 98 (11)a,c | 100 (12)b | <.001 |
| Previous FHCVD | 15.2 | 15.3 | 16.1 | 19.7 | <.001 |
| Smoking | 13.7 | 17.7 | 19.7 | 23.3 | <.001 |
| Diabetes mellitus | 17.2 | 25.8 | 23.7 | 33.0 | <.001 |
| Blood glucose, mean (SD), mg/dL | 102 (25) | 108 (31)a | 110 (32)a,c | 118 (40)b | <.001 |
| Total cholesterol, mean (SD), mg/dL | 204 (36) | 199 (38) | 226 (40)b | 205 (41) | <.001 |
| HDL-C, mean (SD), mg/dL | 58.4 (11.9) | 37.8 (4.9) | 52.2 (9.4) | 35.9 (5.2) | <.001 |
| LDL-C, mean (SD), mg/dL | 126 (33) | 129 (36) | 134 (36)b | 126 (37) | <.001 |
| Triglycerides, mean (SD), mg/dL | 96 (27) | 108 (26) | 205 (68) | 237 (96) | <.001 |
| Metabolic syndrome | 61.2 | 71.3 | 95.9 | 82.1 | <.001 |
| Creatinine, mean (SD), mg/dL | 0.94 (0.37) | 0.98 (0.40)a | 0.98 (0.35)a | 1.01 (0.40)b | <.001 |
| EGFR, mean (SD), mL/min/1.73 m2 | 75.7 (22.9) | 73.7 (24.1)a | 73.5 (23.6)a | 72.4 (24.9)a | <.001 |
| EGFR < 60, mL/min/1.73 m2 | 11.4 | 15.5 | 14.5 | 17.6 | <.001 |
| UAE, mg/g | 5 [2-13.3] | 6.2 [2.8-20]a | 5.6 [2.1-18]a | 8 [3.1-32] | <.001 |
| UAE > 30, mg/g | 13.1 | 18.9 | 17.7 | 25.7 | <.001 |
| ECG-LVH | 2.9 | 3.9 | 3.8 | 3.9 | .001 |
| Previous CVD | 8.4 | 12.5 | 8.7 | 12.7 | <.001 |
AD, atherogenic dyslipidemia; BMI, body mass index; CVD, cardiovascular disease; ECG, electrocardiogram; EGFR, estimated glomerular filtration rate; FHCVD, family history of cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, high-density lipoprotein cholesterol; LVH, left ventricular hypertrophy; UAE, urinary albumin excretion; WC, waist circumference.
Data are expressed as No. (%), mean (standard deviation), or median [interquartile range].
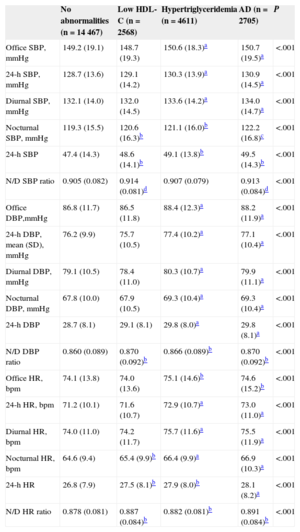
Table 2 shows differences between the 4 groups in office and ABPM heart rate and BP values according to lipid abnormalities. Patients with AD had significantly higher office, 24-h, diurnal, and nocturnal BPand heart rate values, a smaller reduction in nocturnal BP and heart rate values, and increased short-term variability (24-hour standard deviation). Patients with either low HDL-C or hypertriglyceridemia had intermediate values (higher than patients without abnormalities but lower than those with AD). In general, patients with hypertriglyceridemia had higher levels than those with only low HDL-C.
Office, 24-h, Diurnal, and Nocturnal Blood Pressure and Heart Rate, Variability, and Nocturnal Blood Pressure Dipping According to the Presence of Atherogenic Dyslipidemia or any of its Components
| No abnormalities (n=14 467) | Low HDL-C (n=2568) | Hypertriglyceridemia (n=4611) | AD (n=2705) | P | |
|---|---|---|---|---|---|
| Office SBP, mmHg | 149.2 (19.1) | 148.7 (19.3) | 150.6 (18.3)a | 150.7 (19.5)a | <.001 |
| 24-h SBP, mmHg | 128.7 (13.6) | 129.1 (14.2) | 130.3 (13.9)a | 130.9 (14.5)a | <.001 |
| Diurnal SBP, mmHg | 132.1 (14.0) | 132.0 (14.5) | 133.6 (14.2)a | 134.0 (14.7)a | <.001 |
| Nocturnal SBP, mmHg | 119.3 (15.5) | 120.6 (16.3)b | 121.1 (16.0)b | 122.2 (16.8)c | <.001 |
| 24-h SBP | 47.4 (14.3) | 48.6 (14.1)b | 49.1 (13.8)b | 49.5 (14.3)b | <.001 |
| N/D SBP ratio | 0.905 (0.082) | 0.914 (0.081)d | 0.907 (0.079) | 0.913 (0.084)d | <.001 |
| Office DBP,mmHg | 86.8 (11.7) | 86.5 (11.8) | 88.4 (12.3)a | 88.2 (11.9)a | <.001 |
| 24-h DBP, mean (SD), mmHg | 76.2 (9.9) | 75.7 (10.5) | 77.4 (10.2)a | 77.1 (10.4)a | <.001 |
| Diurnal DBP, mmHg | 79.1 (10.5) | 78.4 (11.0) | 80.3 (10.7)a | 79.9 (11.1)a | <.001 |
| Nocturnal DBP, mmHg | 67.8 (10.0) | 67.9 (10.5) | 69.3 (10.4)a | 69.3 (10.4)a | <.001 |
| 24-h DBP | 28.7 (8.1) | 29.1 (8.1) | 29.8 (8.0)a | 29.8 (8.1)a | <.001 |
| N/D DBP ratio | 0.860 (0.089) | 0.870 (0.092)b | 0.866 (0.089)b | 0.870 (0.092)b | <.001 |
| Office HR, bpm | 74.1 (13.8) | 74.0 (13.6) | 75.1 (14.6)b | 74.6 (15.2)b | <.001 |
| 24-h HR, bpm | 71.2 (10.1) | 71.6 (10.7) | 72.9 (10.7)a | 73.0 (11.0)a | <.001 |
| Diurnal HR, bpm | 74.0 (11.0) | 74.2 (11.7) | 75.7 (11.6)a | 75.5 (11.9)a | <.001 |
| Nocturnal HR, bpm | 64.6 (9.4) | 65.4 (9.9)b | 66.4 (9.9)a | 66.9 (10.3)a | <.001 |
| 24-h HR | 26.8 (7.9) | 27.5 (8.1)b | 27.9 (8.0)b | 28.1 (8.2)a | <.001 |
| N/D HR ratio | 0.878 (0.081) | 0.887 (0.084)b | 0.882 (0.081)b | 0.891 (0.084)b | <.001 |
AD, atherogenic dyslipidemia; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; HR, heart rate; N/D, nocturnal/diurnal; SBP, systolic blood pressure; SD, standard deviation.
Data are expressed as mean (SD).
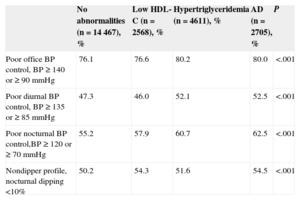
Patients with lipid abnormalities also had worse office BP control (≥ 140 or 90mmHg) and worse ABPM control in the diurnal period (≥ 135 or 85mmHg) and nocturnal period (≥ 120 or 70mmHg). This group also had the highest percentage of nondippers (nocturnal BP < 10%; Table 3). Logistic regression adjusted for age, sex, and the number of antihypertensive drugs was used to assess the association between these lipid abnormalities, poor BP control, and circadian abnormalities (Figure 1). All abnormalities were associated with poor nocturnal BP control and nocturnal nondipping. Only hypertriglyceridemia and AD were associated with poorly controlled office or diurnal BP.
Distribution of Patients With Poor Office Blood Pressure Control, Poor Diurnal and Nocturnal ABPM Blood Pressure Control, and Abnormal Circadian Profile According to Lipid Abnormalities
| No abnormalities (n=14 467), % | Low HDL-C (n=2568), % | Hypertriglyceridemia (n=4611), % | AD (n=2705), % | P | |
|---|---|---|---|---|---|
| Poor office BP control, BP ≥ 140 or ≥ 90 mmHg | 76.1 | 76.6 | 80.2 | 80.0 | <.001 |
| Poor diurnal BP control, BP ≥ 135 or ≥ 85 mmHg | 47.3 | 46.0 | 52.1 | 52.5 | <.001 |
| Poor nocturnal BP control,BP ≥ 120 or ≥ 70 mmHg | 55.2 | 57.9 | 60.7 | 62.5 | <.001 |
| Nondipper profile, nocturnal dipping <10% | 50.2 | 54.3 | 51.6 | 54.5 | <.001 |
AD, atherogenic dyslipidemia; BP, blood pressure; HDL-C, high-density lipoprotein cholesterol.
Association of lipid disorders (low HDL-C, hypertriglyceridemia, and AD) with poor office blood pressure control, poor diurnal and nocturnal ABPM blood pressure control, and decreased nocturnal systolic blood pressure (<10%; nondipping). Values adjusted for age, sex, and number of drugs. ABPM, ambulatory blood pressure monitoring; AD, atherogenic dyslipidemia; 95%CI, 95% confidence interval; HDL-C, high density lipoprotein cholesterol; OR, odds ratio; TG, triglycerides.
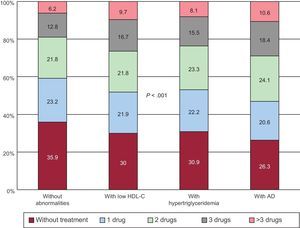
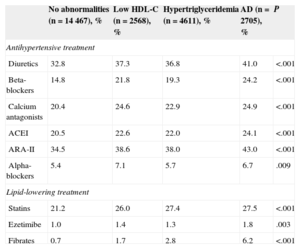
Finally, the use of antihypertensive drugs was assessed in the 4 groups of patients. Figure 2 shows the percentage of patients without antihypertensive treatment and patients taking 1, 2, 3, or more than 3 drugs. As shown, patients with AD received more drugs (2 or more) than the other groups. Table 4 shows the percentage of antihypertensive and lipid-lowering drugs taken by each group. Both classes of drugs were used more frequently in AD patients and there were no differences in drug distribution between the 4 groups.
Antihypertensive Drug Classes Used in the 4 Groups of Patients According to Lipid Abnormalities
| No abnormalities (n=14 467), % | Low HDL-C (n=2568), % | Hypertriglyceridemia (n=4611), % | AD (n=2705), % | P | |
|---|---|---|---|---|---|
| Antihypertensive treatment | |||||
| Diuretics | 32.8 | 37.3 | 36.8 | 41.0 | <.001 |
| Beta-blockers | 14.8 | 21.8 | 19.3 | 24.2 | <.001 |
| Calcium antagonists | 20.4 | 24.6 | 22.9 | 24.9 | <.001 |
| ACEI | 20.5 | 22.6 | 22.0 | 24.1 | <.001 |
| ARA-II | 34.5 | 38.6 | 38.0 | 43.0 | <.001 |
| Alpha-blockers | 5.4 | 7.1 | 5.7 | 6.7 | .009 |
| Lipid-lowering treatment | |||||
| Statins | 21.2 | 26.0 | 27.4 | 27.5 | <.001 |
| Ezetimibe | 1.0 | 1.4 | 1.3 | 1.8 | .003 |
| Fibrates | 0.7 | 1.7 | 2.8 | 6.2 | <.001 |
ACEI, angiotensin-converting enzyme inhibitors; AD, atherogenic dyslipidemia; ARA-II, angiotensin II receptor antagonists; HDL-C, high-density lipoprotein cholesterol.
This study assessed the prevalence of AD and its component lipid disorders in hypertensive patients with or without antihypertensive treatment included in the Spanish ABPM Registry, which is the most extensive database of patients with ABPM monitoring. The combination of high triglyceride and low HDL-C levels (atherogenic dyslipidemia) was found in 11% of the patients. These patients were characterized by a high risk profile that included other risk factors, metabolic or nonmetabolic, target organ damage, and a history of cardiovascular disease. This group also had poor ABPM and office BP, high nocturnal BP, and nocturnal nondipping, which were clearly associated with a poor cardiovascular risk profile.17,18
Atherogenic dyslipidemia is a metabolic disorder mainly caused by insulin resistance, which in most patients is caused by the excessive accumulation of visceral fat, and is considered to be a component of metabolic syndrome.8 Abdominal obesity and metabolic syndrome are common among hypertensive patients in Spain,19,20 but these disorders are not always accompanied by lipid abnormalities. Although 67% of patients in this series met the currently accepted criteria for metabolic syndrome,21 lipid abnormalities were only found in 30% (hypertriglyceridemia) and 22% (low HDL-C levels) of patients. Both abnormalities were present in 11% of patients. However, the clinical features of these patients are of greater interest than the prevalence data. Although the patients were relatively young and mainly male, they had a poor cardiovascular risk profile not only due to other metabolic risk factors, which would be expected, but also to silent target organ damage with a higher prevalence of microalbuminuria, impaired kidney function, and electrocardiographic left ventricular hypertrophy. There was also a high percentage of patients with established cardiovascular disease in this group. They also had high ABPM and office BP,nocturnal nondipping, and decreased short-term variability (24-h BP standard deviation), despite taking a greater number of antihypertensive drugs than the other groups.
The importance of AD is shown by the poor BP control and increased organ damage in AD patients, yet this disorder does not receive the same level of attention as high LDL-C levels. The risk associated with high LDL-C levels is very well established and statin therapy is perceived as highly effective in reducing the risk of events in both primary and secondary prevention.22 In contrast, the presence of AD is less well established as a cause of disease and data on its treatment remain inconclusive.23Most clinical guidelines recommend statin treatment in AD patients at high cardiovascular risk, despite the relatively poor capacity of statins to modify abnormal lipid levels in patients with AD.22 Other classes of drugs that can modify triglyceride and HDL-C levels, such as fibrates, nicotinic acid, or omega-3 fatty acids derivatives, are only considered for use when LDL-C or non-HDL cholesterol levels cannot be modified by statin treatment.
In the AD group, treatment with statins was the most common lipid-lowering therapy (28% vs 23% the other groups). In contrast, only 6.2% (1.3% in the other groups) received fibrate monotherapy and 1.4% (0.3% in the other groups) received statin-fibrate combination therapy. The underuse of these drugs could be because AD patients are considered to be at low risk or because of their perceived poor efficacy.
This study is limited by its cross-sectional design and because it is based on registry data, and thus it did not investigate the effect of past or present treatment on the onset of lipid abnormalities or on BP values and organ damage. Moreover, bias in group selection (more lipid abnormalities or better control of lipid parameters) may have been caused by the inclusion of patients with only complete laboratory data on lipid profile. Thus, the sample may not accurately reflect the general characteristics of the patients included in the registry.
CONCLUSIONSIn total, 11% of hypertensive patients included in the Spanish ABPM Registry had AD. This group of patients had a poor risk profile characterized by increased target organ damage (cardiac and renal) and a greater percentage of established cardiovascular disease. Patients with AD had poor ABPM and office BP control, despite the greater use of antihypertensive drugs. In addition, the use of lipid-lowering drugs was limited, especially in combination therapy. Greater therapeutic effort is needed to reduce overall risk in these patients.
CONFLICTS OF INTERESTThe authors declare having received fees for participating in continuing medical education activities sponsored by Lacer, SA.
The Spanish ABPM Registry MAP was initiated and maintained by unrestricted funding from Lacer SA and received partial funding from the Ministry of Economy and Competitiveness (PI10/01011) and the Spanish Society of Hypertension-Spanish League for the Control of Hypertension (SEH-LELHA).