In recent years, imaging techniques have revolutionized the diagnosis of heart failure. In patients with a clinical picture of acute decompensation, prognosis is largely determined by early implementation of general measures and treatment of the underlying cause. Given its diagnostic yield and portability, ultrasound has become an essential tool in the setting of acute heart failure, and is currently found in all medical departments involved in the care of the critically ill patient. Cardiac magnetic resonance and computed tomography allow detailed characterization of multiple aspects of cardiac structure and function that were previously unavailable. This helps guide and monitor many of the treatment decisions in the acute heart failure population in an entirely noninvasive way. This article aims to review the usefulness of the imaging techniques that are clinically relevant in the context of an episode of acute heart failure. We discuss the indications and limitations of these techniques in detail and describe the general principles for the appropriate interpretation of results.
Keywords
Heart failure (HF) is one of the leading causes of morbidity and mortality in western countries.1 This high prevalence explains why HF is responsible for most emergency department consultations for dyspnea, be it new onset HF, or acute decompensation of HF in patients with chronic disease.2 The severity of decompensation can vary, from a worsening of exertional dyspnea, to acute pulmonary edema or cardiogenic shock.3
Over the years, imaging techniques have acquired a fundamental role in patients with HF.4 Due to its diagnostic yield, absence of risk, versatility, and portability, ultrasound is the first-line bedside tool for patients with acute HF. Ultrasound has been extensively validated in acute HF patients for determination of the underlying cause, hemodynamic assessment, therapeutic guidance, and prognostic stratification.5 Furthermore, in recent years, the reduction in size and cost of ultrasound equipment has allowed the technique to spread beyond the specialty of cardiology. Ultrasound is now used routinely in the assessment and classification of patients with dyspnea in the emergency department and intensive care unit.6,7
Transthoracic echocardiography is the essential tool in the assessment of structural and functional changes that cause or are associated with HF.3 In some clinical scenarios, transesophageal echocardiography, 3-dimensional echocardiography, and myocardial deformation techniques can offer additional information once the patient has been stabilized. Computed tomography (CT) has a more valuable role in acutely ill patients who present with symptoms of dyspnea and chest pain.8 Magnetic resonance imaging (MRI) gives essential information on the nature and extent of associated myocardial damage.
In this article, we review the value and limitations of cardiac imaging techniques in the context of patients with acute HF, with particular emphasis on the indications for each technique, and provide some general principles for the appropriate interpretation of their results (Table).
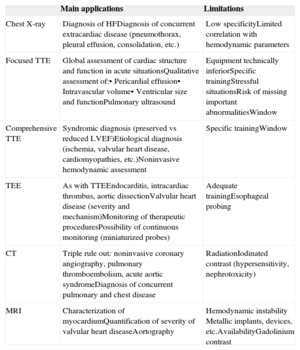
Main Applications and Limitations of Cardiac Imaging Techniques in the Diagnosis and Management of Patients With Acute Heart Failure
| Main applications | Limitations | |
|---|---|---|
| Chest X-ray | Diagnosis of HFDiagnosis of concurrent extracardiac disease (pneumothorax, pleural effusion, consolidation, etc.) | Low specificityLimited correlation with hemodynamic parameters |
| Focused TTE | Global assessment of cardiac structure and function in acute situationsQualitative assessment of:• Pericardial effusion• Intravascular volume• Ventricular size and functionPulmonary ultrasound | Equipment technically inferiorSpecific trainingStressful situationsRisk of missing important abnormalitiesWindow |
| Comprehensive TTE | Syndromic diagnosis (preserved vs reduced LVEF)Etiological diagnosis (ischemia, valvular heart disease, cardiomyopathies, etc.)Noninvasive hemodynamic assessment | Specific trainingWindow |
| TEE | As with TTEEndocarditis, intracardiac thrombus, aortic dissectionValvular heart disease (severity and mechanism)Monitoring of therapeutic proceduresPossibility of continuous monitoring (miniaturized probes) | Adequate trainingEsophageal probing |
| CT | Triple rule out: noninvasive coronary angiography, pulmonary thromboembolism, acute aortic syndromeDiagnosis of concurrent pulmonary and chest disease | RadiationIodinated contrast (hypersensitivity, nephrotoxicity) |
| MRI | Characterization of myocardiumQuantification of severity of valvular heart diseaseAortography | Hemodynamic instability Metallic implants, devices, etc.AvailabilityGadolinium contrast |
CT, computed tomography; HF, heart failure; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Early focused treatment for acute HF has been shown to have a major impact on length of hospital stay9 and in-hospital mortality.10 The history and physical examination have limited yield at the time of admission of the acute patient, due to the nonspecific symptoms of HF and frequent comorbidity. Whilst natriuretic peptides help to classify HF patients,11 there is an extensive grey area that needs more techniques. Conventional chest X-ray is an absolute indication for all patients with suspected or diagnosed HF of recent onset.11 Although chest X-ray sensitivity is relatively low, it does show signs specific to HF (such as vascular redistribution, septal lines, and alveolar edema) and helps to exclude alternative extracardiac diagnoses.
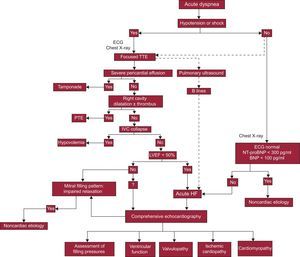
When acute HF is suspected, a transthoracic echocardiogram is recommended and should be done immediately if there is hemodynamic instability (Figure 1).3,11–13 There is a growing tendency for the dyspneic patient's point of care physician to perform the initial ultrasound examination.14 The aims and method of this general ultrasound (focused, or point-of-care ultrasound) are well established in specific documents, and it does not replace controlled echocardiography interpreted by a cardiologist (comprehensive echocardiography).7,13 The aims of the initial ultrasound examination are to rule out pericardial effusion, evaluate biventricular function, and estimate blood volume by visualization of the inferior vena cava (IVC) (Figure 1).5 Qualitative identification of left ventricular systolic dysfunction and noncollapsible IVC in this way have shown high specificity for diagnosis of acute HF.15 For patients with suspected acute HF who are hemodynamically stable, determination of natriuretic peptides is considered an alternative to immediate echocardiography,3 but the strategy based on early echocardiography has been shown to be faster and more specific.16
Diagnostic algorithm for heart failure. BNP, brain natriuretic peptide; ECG, electrocardiogram; HF, heart failure; IVC, inferior vena cava; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; PTE, pulmonary thromboembolism; TTE, transthoracic echocardiography.
In addition, all conventional ultrasound machines allow pulmonary examination, providing useful etiological information in acute respiratory failure. The presence of pulmonary edema, pneumothorax, or pleural effusion can be identified with high sensitivity and specificity using this technique.5 The echographic image of interstitial pulmonary edema (B lines) consists of bilateral hyperechoic linear structures that begin at the pleura and are shaped like “comet-tails”. They are a product of interlobular septal thickening and alveolar occupation. This echographic pattern correlates with the values of pulmonary capillary pressure and pulmonary extravascular volume.17
After this initial ultrasound examination, all patients with a diagnosis of acute HF should have a comprehensive echocardiogram. This allows the medical team to establish a definitive diagnosis, characterize associated lesions, assess hemodynamic status, and obtain relevant information to determine the prognosis. Repeat echocardiography is indicated if the clinical situation changes, or after therapeutic maneuvers that could affect cardiac function.11
Often, dyspnea and chest pain occur together with no specific ECG changes of myocardial ischemia. Even an acute coronary event such as pulmonary embolism or acute aortic syndrome can present in this way, and in all cases, early diagnosis is vitally important. Currently, multidetector CT allows not only high quality coronary angiography, but also aortography and pulmonary angiography, so that just 1 radiological investigation can exclude conditions in these 3 areas (triple rule out).18
ASSESSMENT OF LEFT VENTRICULAR SYSTOLIC FUNCTIONIn clinical practice, the most established index of left ventricular (LV) systolic function is ejection fraction (EF). Patients with HF should be subdivided according to EF, into 2 syndromic groups: those with LV systolic dysfunction (EF < 50%) and those with normal, or “preserved” LV function (EF ≥ 50%).11,19
Several methods are available to calculate EF using echocardiography. The accuracy of visual estimation of EF is excellent in cardiologists experienced in echocardiography,20 and is good in other properly trained health care professionals.13,21 However, use of the biplane volumetric Simpson method is recommended for EF measurement whenever possible.22 Three-dimensional echocardiography provides volume measurement without geometric assumptions, but acquisition and postprocessing depend greatly on image quality, which limits the systematic use of 3-dimensional echocardiography in acute situations. Echocardiography with contrast improves visualization of the endocardial edge, and allows more reliable assessment of EF and detection of abnormal segment contraction. The safety and cost-effectiveness of contrast echocardiography in critically ill patients is well established.23 For patients with no transthoracic ultrasound window (such as those who are mechanically ventilated or in the perioperative period of cardiac surgery, etc.) transesophageal study is the best alternative. Transesophageal study is also the best option in the case of suspected aortic disease, prosthetic valve dysfunction, endocarditis, or atrial thrombi (for which transesophageal echocardiography has higher sensitivity than transthoracic echocardiography).5,24 Magnetic resonance imaging and CT provide precise information on cardiac morphology and function, and are the gold standard for estimation of EF.11 However, measurement of EF is not a routine clinical indication for these investigations in the acute setting (see below).
Irrespective of the technique used, EF has inherent limitations as a measurement of LV systolic function that are particularly important in acute HF. The sensitivity of EF to load conditions (such as blood volume, afterload, heart rate, valve disease, etc.) means that EF must be interpreted as an instantaneous value, obtained in a particular hemodynamic situation. This limitation has led investigators to implement new methods for quantification of LV systolic function that are more robust and less sensitive to the instantaneous load conditions.
Myocardial deformation echocardiography allows both regional and global quantification of myocardial function. Currently, these measurements are fundamentally obtained from postprocessing of 2-dimensional echocardiographic images by following the reflective elements of the myocardial wall (speckle-tracking). The most widely used index of deformation in clinical echocardiography is Lagrangian strain, which is defined as the change in myocardial length in a specific direction (longitudinal, radial, or circumferential) compared with its baseline length. Of these, longitudinal strain obtained in apical planes is the most commonly used index in clinical practice.22–25 Strain rate refers to the rate of change of strain per unit of time and is less sensitive than strain to afterload.26
Several studies have shown the value of these deformation techniques in detecting subclinical myocardial dysfunction. In patients with acute HF and normal EF, abnormal values of radial and longitudinal deformation have been shown to exist in the presence of preserved circumferential function.27 These findings may indicate a certain degree of systolic dysfunction despite EF being within normal limits,28 but could also be related to the different dependencies on load conditions of the 3 spatial components.29 Deformation parameters could also provide offer more prognostic information than EF in patients with acute HF.30
Another approach to the characterization of LV chamber systolic function can be obtained by study of fluid dynamics of the blood within the LV.31 It has long been known that the acceleration capacity of blood during ejection correlates with the maximal elastance at end-systole, a reference parameter for LV systolic function.32 On this basis, our group implemented and validated a method based on postprocessing of color Doppler images in M-mode. The measurement of peak intraventricular systolic gradient obtained in this way is a method that is easily applicable to clinical practice33 and provides precise information on LV function.29,34,35
All these methods aim to measure the contractile function of the LV. Therefore, in a hemodynamically unstable patient, the results of systolic function measurement must always be interpreted in the context of the supportive therapy being administered. Events such as inotropic stimulation, mechanical ventilation, ischemia, or myocardial revascularization always have an immediate effect on any of these indices.11
ETIOLOGICAL DIAGNOSISIschemic CardiopathyAtherosclerotic coronary artery disease can provoke acute HF as a complication of an acute coronary syndrome (ACS) (10%-20% of cases), or due to decompensation of an established chronic ischemic cardiomyopathy.36 Even in the absence of chest pain, myocardial ischemia is the precipitating factor in a quarter of cases of acute decompensation of HF and is often underdiagnosed.36 Identification of coronary artery disease determines the therapeutic management of patients that present with acute HF.
In the third universal definition of myocardial infarction, cardiac imaging was formally incorporated into the diagnostic criteria of acute myocardial infarction. Therefore, the finding of de novo changes in segmental contraction in the context of increased levels of markers of myocardial damage constitutes an acute myocardial infarction.37
In patients presenting with HF and LV systolic dysfunction, it is imperative to rule out possible associated coronary artery disease.11,38 In patients with normal systolic function, the decision to study the coronary anatomy can rely on the presence of clinical angina or a positive ischemia detection test. In acute HF, if the HF is not related to an ACS and there is moderate suspicion of coronary artery disease, CT coronary angiography is the technique of choice to study the coronary tree. This applies to both patients with systolic dysfunction39 and those with normal EF.40 Gadolinium-enhanced cardiac MRI allows identification of viable myocardium41 and prediction of clinical outcome after revascularization in terms of the proportion of viable myocardium (hibernating or stunned).42 It has recently been shown that CT can also be used to characterize the myocardium.43
Imaging techniques play a fundamental role in severe acute HF and in cardiogenic shock in the context of ACS. Ischemic disease of the right ventricle (RV) in isolation is uncommon and is generally associated with an inferior infarct. Echocardiography shows a dilated dysfunctional RV, abnormal movement of the septum, and occasionally, functional tricuspid regurgitation, all in the absence of raised pulmonary pressures. The reduction of preload caused by an RV infarct can mask severe LV dysfunction. These aspects should be taken into consideration whenever ventricular assist options are being evaluated.5
Mechanical complications are another common cause of acute HF in ACS. Although rupture of the free wall of the LV is usually catastrophic, it can occasionally present as a subacute pericardial effusion and progress to pseudoaneurysm formation. Computed tomography or MRI techniques can be useful for the differential diagnosis of subacute rupture after an acute myocardial infarction.44 Interventricular communication after infarction requires urgent surgical intervention and is usually diagnosed with echocardiography. The characteristics of the tissue surrounding the interventricular communication can be assessed with MRI, if allowed by the patient's the clinical status. Acute mitral regurgitation in ACS is usually secondary to ventricular dysfunction and changes in the geometry of the subvalvular apparatus, with apical displacement of the area of coaptation of the mitral valve, and reduction of closing forces. Rarely, mitral regurgitation can be due to rupture of the posterior papillary muscle. In this situation, echocardiography shows a mobile triangular structure attached to the prolapsed mitral leaflet, which enters the left atrium during systole. This mechanical complication is usually characterized in detail using transesophageal echocardiography, during surgery if possible.
Stress Cardiomyopathy and MyocarditisSometimes, patients presenting with acute HF have regional wall motion abnormalities that are not caused by epicardial coronary artery disease. Stress cardiomyopathy is characterized by a transient LV dysfunction secondary to a catecholaminergic release, generally related to an identifiable stress. The typical echocardiographic pattern is akinesia or dyskinesia of the mid and apical segments of all walls, hypercontractility of the basal segments, and ventricular dysfunction to a variable degree. Even if there are echocardiographic signs that suggest stress cardiomyopathy (regional wall motion abnormalities affecting more than 1 coronary artery territory), urgent invasive coronary angiography is required to exclude ACS45.
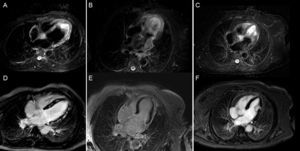
A quarter of patients with stress cardiomyopathy have clinical HF and up to 7% have cardiogenic shock.46,47 After diagnosis, echocardiography allows identification of patients with increased risk of cardiac complications. Factors independently associated with an increased rate of cardiovascular events are the degree of ventricular dysfunction, the presence of increased filling pressures, and the presence of mitral regurgitation. Other factors that determine the clinical severity are LV outflow tract obstruction, the number of affected segments, pulmonary systolic pressure, and the extent of disease.46 Occasionally, the initial diagnosis may be indistinguishable from an embolic event or myocarditis.48 Cardiac MRI allows differential diagnosis investigation in the subacute phase. T2-weighted sequences of stress cardiomyopathy show diffuse edema with transmural distribution corresponding to the dysfunctional myocardial segments, which does not follow a vascular distribution and generally disappears 2 weeks after onset of symptoms. Late gadolinium enhancement is characteristically negative (Figure 2).
Usefulness of cardiac magnetic resonance for differential diagnosis in patients with acute heart failure, raised markers of myocardial injury, and no coronary artery lesions. Images corresponding to T2-STIR sequences (above) and late enhancement after gadolinium administration (below) in 3 patients. A and D: study compatible with acute myocarditis, with marked hyperintensity (edema) in septum and lateral wall (A) and extensive patchy mesocardial late enhancement (D). B and E: study compatible with stress cardiomyopathy, with edema affecting the distal two thirds of all walls (B) and no evidence of late enhancement (E). C and F: study compatible with embolic infarction in the context of a hypertensive emergency with edema localized to the middle third of the lateral wall (C) and focal transmural enhancement in the same location (F).
Rarely, acute myocarditis causes a devastating picture of acute HF and cardiogenic shock. It is estimated that the myocarditides account for 10% of acute unexplained HF presentations.49 The clinical pathology has 2 pictures: fulminant myocarditis and acute myocarditis.50 Fulminant myocarditis is characterized by a clear viral prodrome, a severe lymphocytic inflammatory infiltrate, and a severe deterioration in ventricular function. It usually resolves completely, so if required by the hemodynamic situation, supportive measures are essential. Giant cell myocarditis is an extremely uncommon disease, of autoimmune etiology, with very poor prognosis, and is indistinguishable from the onset of fulminant viral myocarditis.51 Acute myocarditis has a more indolent onset than fulminant myocarditis, often without a clear prodrome, but progresses more often to dilated cardiomyopathy.52 The myocardium characteristically shows patchy infiltration.
The echocardiographic findings, although nonspecific, may show 1 of 2 clinical patterns: a nondilated, dysfunctional LV with increased wall thickness, indicating fulminant myocarditis; or a dilated LV with normal wall thickness, supporting a diagnosis of acute myocarditis.52,53 In both cases, there may be segmental contraction abnormalities and varying degrees of diastolic dysfunction. Also, in both cases it is not uncommon to have intraventricular thrombi and/or a degree of pericardial effusion. Right ventricular dysfunction is uncommon, but its presence is an important predictor of mortality and/or the need for heart transplantation. Monitoring of myocardial formation parameters could be useful to identify those patients most susceptible to developing dilated cardiomyopathy.54
Due to the ability of MRI to detect myocardial inflammation, it is the technique of choice for the differential diagnosis of myocarditis (Figure 2).55 This imaging modality is currently indicated for symptomatic patients with evidence of acute myocardial injury and suspected viral etiology.56 The MRI criteria for myocarditis are increased myocardial signal on T2-weighted sequences (edema), early enhancement after gadolinium administration (hyperemia and increased capillary permeability), and/or the presence of late enhancement (necrosis-fibrosis). A positive result in any 2 of these 3 criteria has a diagnostic accuracy of 79%.55
CardiomyopathiesAcute HF can be the mode of presentation of alcoholic or toxic dilated cardiomyopathy, Chagas disease, or a primary cardiomyopathy. Initial etiological orientation can be carried out based on age, cardiovascular risk factors, a history of toxic substance ingestion, and the geographical origin of the patient.
It is unusual for hypertrophic cardiomyopathy to present as acute HF. When it does, it may be precipitated by tachyarrhythmias, acute mitral regurgitation (cord rupture or endocarditis), myocardial ischemia, or comorbidity (such as anemia or hyperthyroidism, etc.).57 In this context, echocardiography is essential to establish if there is an intraventricular obstruction, to locate and monitor the severity of the obstruction, and to assess the degree and mechanism of mitral regurgitation. An infiltrative cardiomyopathy-generally secondary to amyloidosis-should be suspected in the case of a hypertrophic LV with a normal EF, abnormal wall echogenicity, a restrictive filling pattern and frank reduction of longitudinal shortening.5,58 In patients with a primary cardiomyopathy of probable genetic origin or a suspected storage disease, MRI is an essential technique and should always be included in the diagnostic algorithm.
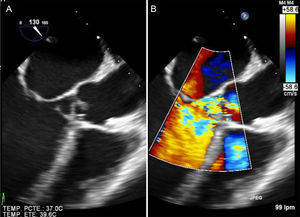
Valvular heart diseaseAcute severe HF of valvular cause is usually secondary to severe left-sided valvular heart disease or prosthetic valve dysfunction.5 The loss of normal anatomy of the mitral valve apparatus, generally secondary to endocarditis, can be catastrophic. Although transthoracic echocardiography can be diagnostic, a transesophageal study is required after initial stabilization of the patient (Figure 3).
Acute HF in a patient with rheumatic mitral stenosis is usually precipitated by atrial fibrillation or by an increase in circulating volume (for example in pregnancy). Acute HF in patients with prosthetic mitral valves always requires exclusion of prosthetic dysfunction; the most feared causes are prosthetic thrombosis and endocarditis. A transesophageal echocardiogram usually confirms the diagnosis, but study of the prosthetic mechanism with fluoroscopy or CT can also be useful. Aortic regurgitation can cause severe HF, generally if the AR is of sudden onset. In this case, aortic dissection must be ruled out with a CT, and endocarditis ruled out with transesophageal echocardiography (Figure 3).5,59,60
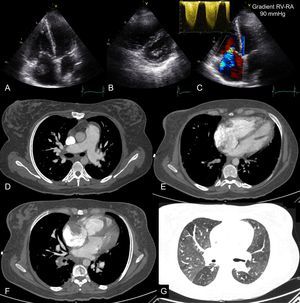
Acute Right Heart FailurePulmonary thromboembolism may not cause chest pain and may have isolated signs of acute severe right HF (dyspnea, low cardiac output, and signs of increased central venous pressure). Computed tomography pulmonary angiography is the current investigation of choice to visualize the pulmonary vascular tree and exclude pulmonary thromboembolism. However, in patients with dyspnea and sudden hemodynamic instability, urgent transthoracic echocardiography performs well. In this situation, acute RV pressure overload causes RV dilatation and dysfunction, which is usually easy to identify. Conversely, a normal RV practically rules out massive pulmonary thromboembolism. Transthoracic echocardiography is particularly relevant because, in the presence of shock, RV pressure overload warrants reperfusion therapy, even in the absence of radiological study.61 Although very uncommon, direct visualization of a thrombus in the right cavities establishes a diagnosis of pulmonary thromboembolism and implies increased early mortality62 (Figure 4).
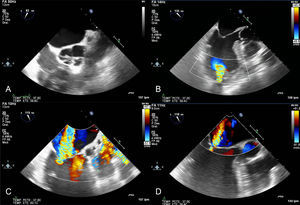
Echocardiography and computed tomography in a patient with acute pulmonary thromboembolism and chronic thromboembolic disease. Images from transthoracic echocardiography in apical planes (A and C) and short axis parasternal planes (B) showing severe dilatation of the right cavities and image of thrombus in right atrium. The continuous spectrum Doppler of tricuspid regurgitation is compatible with severe pulmonary hypertension (C). D: pulmonary angiography showing filling defects in segmental pulmonary branches compatible with acute pulmonary thromboembolism and dilatation of pulmonary arterial trunk as a sign of precapillary pulmonary hypertension. E: 4-chamber axial plane showing severe left ventricular dilatation, with an right ventricle/left ventricle ratio > 1. F: imaging compatible with right atrium thrombus. G: mosaic attenuation pattern of pulmonary parenchyma as a sign of precapillary pulmonary hypertension. RA, right atrium, RV, right ventricle.
In hemodynamically stable patients with pulmonary thromboembolism, dilatation and dysfunction of the RV have prognostic value.63 Using echocardiography, a right-to-left shunt through a patent foramen ovale can also be detected.61 Furthermore, echocardiography allows detection of signs of chronic RV overload such as RV hypertrophy, or indirect signs of chronic thromboembolic pulmonary hypertension (Figure 4).
NONINVASIVE MONITORINGAssessment of Hemodynamic StatusIn patients with acute HF, hemodynamic monitoring techniques are often used until the patient is clinically stable. However, there is no evidence that invasive monitoring with Swan-Ganz catheters in patients with acute HF has an impact on survival.64 Therefore, in these patients, conventional Doppler echocardiography has been proposed as a noninvasive, affordable tool for hemodynamic assessment.65,66 Disposable monoplane transesophageal echocardiography probes allow qualitative image-based monitoring, and preliminary results are promising.67,68
The hemodynamic parameters that are usually monitored using combined echocardiography-Doppler techniques are right atrial pressure (RAP), systolic and diastolic function of the LV, LV filling pressures, stroke volume, and pulmonary pressures. These basic measurements can be used to derive indices such as cardiac output and vascular resistance, etc.
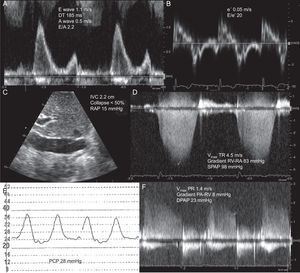
Right Atrial PressureMeasurement of the IVC diameter and collapsibility index allows fast, direct estimation of RAP.69,70 An increase in RAP dilates the IVC such that an IVC diameter of > 2 cm correlates with a RAP > 10mmHg.71 During respiratory movements, transmission of thoracic pressure during inspiration causes a partial collapse of the IVC of between 35% and 50%. With a greater, short inspiratory effort (sniff), IVC collapse exceeds 50%. Regarding the diameter and degree of IVC collapse, some authors have set specific RAP values.70 In patients connected to mechanical ventilation, evaluation of the size and collapsibility of IVC is not possible, but a collapsed IVC indicates hypovolemia. For this group of patients, it can be useful to determine the flow pattern in the hepatic veins through pulsed Doppler. In normal conditions, the systolic forward flow is greater than the diastolic forward flow, but when RAP is high, the preponderance of the systolic wave is lost (S < D).72
Diastolic Function and Assessment of Filling PressuresDiastolic dysfunction is the main cause of raised PCP in patients with HF and normal EF. There are various mechanisms that can lead to diastolic dysfunction in these patients, which means that the atrioventricular gradient responsible for filling can be altered as a consequence of: a) slowed relaxation; b) loss of suction generated by elastic recoil; c) increase in myocardial stiffness, and/or d) increased ventricular operating volumes due to inadequate blood volume management. These same mechanisms can also increase left atrial pressure in patients with systolic dysfunction.
Precise assessment of the contribution made by each of these 4 mechanisms to diastolic dysfunction in an individual patient is only possible with analysis of the pressure-volume curves obtained with high fidelity catheters and preload intervention.73,74 The complexity of this technique in clinical practice has led investigators to seek noninvasive imaging-based approaches. Of these approaches, transmitral pulsed Doppler combined with pulsed tissue Doppler of the mitral annulus provides very useful information for clinical decision-making in patients with HF.65 Usually, several echocardiographic parameters are combined to estimate LV diastolic function and filling pressures.
The velocity of early mitral filling (E wave) depends fundamentally on the left atrial pressure and the degree of LV depressurization (relaxation and elastic recoil). The deceleration time of early filling is determined by the operative ventricular stiffness. The velocity of late mitral filling (A wave) is determined by the atrial contractile function and ventricular stiffness. Early diastolic mitral annular velocity (e’) is also particularly useful. Although impaired relaxation is hightly dependent on the atrioventricular pressure gradient, it delays and reduces mitral annular longitudinal movement. On this basis, the E/e’ ratio has been proposed as an indicator of raised left filling pressures.
In the presence of LV systolic dysfunction, restrictive filling (E/A ratio ¿ 2 with deceleration time ¿ 150ms) indicates a raised left atrial pressure. In contrast, an impaired relaxation filling pattern (E/A ¿ 1, E ¿ 50cm/s) indicates normal left filling pressures. In patients with known HF, an E/A ratio ¿ 1 is considered a good marker of adequately optimized treatment. In this regard, changes in transmitral pattern obtained with various interventions have been proposed as possible prognostic markers in patients with acute HF. Thus, a reversible restrictive pattern after infusion of nitroprusside identifies a group of patients with a low event rate and better response and tolerance to beta-blockers.75
For the group of patients whose E/A ratio is between 1 and 2, other Doppler parameters are recommended.76 The most widely used parameter is the E/e’ ratio, given that study of pulmonary venous flow has little value in the acute setting. In patients with systolic dysfunction and acute HF, an E/e’ ratio > 15 predicts a pulmonary capillary pressure > 15mmHg.65 The predictive value is worse in patients with advanced HF, ventricular remodeling, significant mitral regurgitation, or asynchrony, and in patients with resynchronization devices.77 However, the E/e’ ratio has been validated as a reliable method of monitoring pulmonary capillary pressure during therapeutic interventions.65
In patients with normal systolic function, there is a poor correlation between transmitral filling parameters and hemodynamic parameters. Therefore, the diagnosis of HF with normal EF usually requires an E/e’ ratio ≥ 15 to be shown (Figure 5). If the value of E/e’ ranges between 8 and 15, other parameters are required to support the diagnosis of increased left atrial pressure.76
Estimation of left filling pressures using Doppler echocardiography in a patient with dyspnea and normal left ventricular function. A: pulsed-Doppler of mitral filling showing a restrictive filling pattern. B: tissue Doppler of lateral mitral annulus, allowing estimation of E/e’ ratio > 15. C: Subcostal plane of inferior vena cava. D: continuous Doppler of tricuspid regurgitation and estimation of pulmonary systolic pressure. E: pulmonary capillary pressure signal obtained with Swan-Ganz catheter. F and G: Doppler of pulmonary regurgitation and estimation of diastolic pulmonary pressure. DPAP, diastolic pulmonary artery pressure; DT, deceleration time of early mitral filling; IVC, inferior vena cava; PA, pulmonary artery; PCP, pulmonary capillary pressure; PR, pulmonary regurgitation; RA, right atrium; RAP, right atrial pressure; RV, right ventricle; SPAP, systolic pulmonary artery pressure; TR, tricuspid regurgitation; Vmax, maximal velocity. A wave, maximal velocity of late (atrial) mitral filling. E wave, maximal velocity of early mitral filling. e’ wave, protodiastolic velocity of lateral mitral annulus.
In patients on mechanical ventilation, the mitral filling pattern and assessment of E/e’ ratio can help in the differential diagnosis between HF and respiratory distress syndrome and help predict the success of withdrawal of ventilatory support measures.78,79
With this evidence base, the European and American societies of echocardiography have established a complete algorithm for the estimation of left filling pressures76 (Figure 1) Although this algorithm has been acceptably validated in patients with acute HF, more accurate alternatives continue to be explored.65 Global protodiastolic strain seems to provide more accurate estimation than e’ of left atrial pressure.80 In the same way, the reverse pressure difference between the apex and LV outflow tract can increase the accuracy of filling pressure estimation.81 However, the value of these alternative indices in the acute setting remains to be shown.
Calculation of Stroke Volume, Cardiac Output, and Systemic ResistanceThe most reliable method for estimating LV stroke volume using ultrasound is based on measurements of the velocity-time integral at the level of the LV outflow tract and the section area at this same point. This methodology is feasible in the setting of acute HF and correlates well with invasive measurements. Using cardiac output, estimated RAP, and mean arterial pressure from sphygmomanometry, it is possible to calculate systemic vascular resistance.65
Pulmonary Artery PressuresContinuous wave Doppler spectrograms of tricuspid regurgitation and pulmonary regurgitation allow approximation of systolic and diastolic pulmonary pressures, respectively. In the absence of pulmonary and/or hepatic disease, raised systolic pulmonary pressure signifies raised left atrial pressure.82 If diastolic pulmonary pressure can be obtained, it can be used as a parameter instead of pulmonary capillary pressure (Figure 5).83
Diagnosis of Complications After Invasive ProceduresAfter a surgical intervention, the onset of acute HF or hemodynamic instability requires urgent investigation. Generally, the technique of choice is transesophageal echocardiography aimed at assessing biventricular function and ruling out pericardial effusion.
After a percutaneous procedure, the most common complication is cardiac tamponade, which is easily diagnosed with transthoracic echocardiography. However, after structural procedures, hemodynamic instability requires assessment of normoposition of the implanted device (Figure 6).84 Migration of a transcatheter aortic prosthesis, migration of closure devices for interatrial communications or atrial appendage closure, and mitral plication can all cause severe obstruction of the mitral valve or the LV outflow tract. Transcatheter aortic prosthesis migration can cause massive aortic regurgitation.85
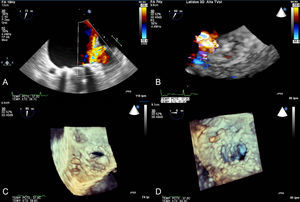
Patient with mechanical mitral prosthesis who was admitted for acute heart failure and increased transprosthetic mitral gradient. Transesophageal study showed severe paravalvular mitral regurgitation at the anteroseptal level. A: 2-dimensional color Doppler imaging in midesophageal plane. B: 3-dimensional color Doppler echocardiography imaging in real time; percutaneous closure was monitored using 3-dimensional transesophageal echocardiography in real time. The sheath can be seen crossing the paravalvular dehiscence (C). The moment in which the device is about to be released (D) can also be seen.
Extracorporeal circulation therapies and ventricular assist therapies are used increasingly in critically ill patients, both as a bridge to recovery or transplantation and as a definitive treatment. In this context, echocardiography has become an essential tool for selecting candidate patients, choosing the type of support required, optimizing support, and assessing its withdrawal or weaning.5,86
In candidates for ventricular assist devices, the status of RV function determines the need for univentricular or biventricular device implantation.87 Once implanted, echocardiography-generally transesophageal-should be used to optimize the device parameters, to confirm its position in the central septum, to confirm intermittent opening of the aortic valve, and to confirm mild or reduced mitral regurgitation, which signifies that the LV is adequately unloaded (Figure 7). Dynamic determination of the LV dimensions and valve function using echocardiography during ramp protocols allows detection of thrombotic complications.88
Transesophageal study in a patient with Berlin Heart Excor® biventricular assist device with aortic valve cerclage. A: short axis of aortic valve. B: cannula positioned in the apex of the left ventricle with no evidence of adjacent thrombosis. C: color Doppler at the level of the mitral valve showing severe (IV/IV) functional mitral regurgitation due to annulus dilatation. After adjusting the device parameters, the mitral regurgitation was partially corrected (D).
As in other modern cardiology settings, imaging techniques are indispensable for clinical decision-making in patients with acute HF. It is only through knowledge of the indications, value, and limitations of imaging techniques that clinicians can adequately assess the etiology of the clinical presentation, assess its severity, guide treatment, and establish the prognosis of patients with acute HF.
FUNDINGThis study was funded by the Carlos III Institute of Health Cardiovascular Research Network, Madrid, Spain (RD12/0042), PI12/02885 and CM12/00273 (Red de Investigación Cardiovascular del Instituto de Salud Carlos III), and by the Gregorio Marañon Biomedical Research Foundation (Fundación de Investigación Biomédica Gregorio Marañón).