According to the current European Society of Cardiology guidelines for patients with atrial fibrillation (AF), oral anticoagulation should be prescribed when the CHA2DS2-VASc1 score is greater than or equal to 2, provided that the patient does not have mitral stenosis, mechanical prostheses, or hypertrophic cardiomyopathy. This treatment is not recommended for men scoring 0 or women scoring 1.2 As patient age is a factor incorporated in this scale (1 point is added for age > 65 years), a dilemma may arise when deciding whether to administer coagulation to patients approaching the age of 65 years who have had an episode of AF.
With the aim of analyzing the impact of starting oral anticoagulation at 65 years of age, we used data from the Cardio CHUVI-AF registry.3,4 From January 2014 to January 2018, the registry included 16 202 patients with a diagnosis of AF in the Vigo health care area. The study was approved by the local ethics committee, and because of its retrospective and anonymized nature, the researchers were exempted from obtaining informed consent from participants. Patients with mechanical prostheses, moderate-severe mitral stenosis, or hypertrophic cardiomyopathy were excluded. All patients with a CHA2DS2-VASc score of 0 for men and 1 for women (n=861; 5.3% of the total) were identified, and those reaching the age of 65 years during the follow-up period were included. Thus, the study population consisted of 389 AF patients whose CHA2DS2-VASc score during follow-up (8.1±1.5 years) increased from 0 to 1 point (in the case of men) or from 1 to 2 points (in the case of women), because they reached the age of 65 years. The mean age of these patients was 60.9±2.7 years and 149 (38.3%) were women.
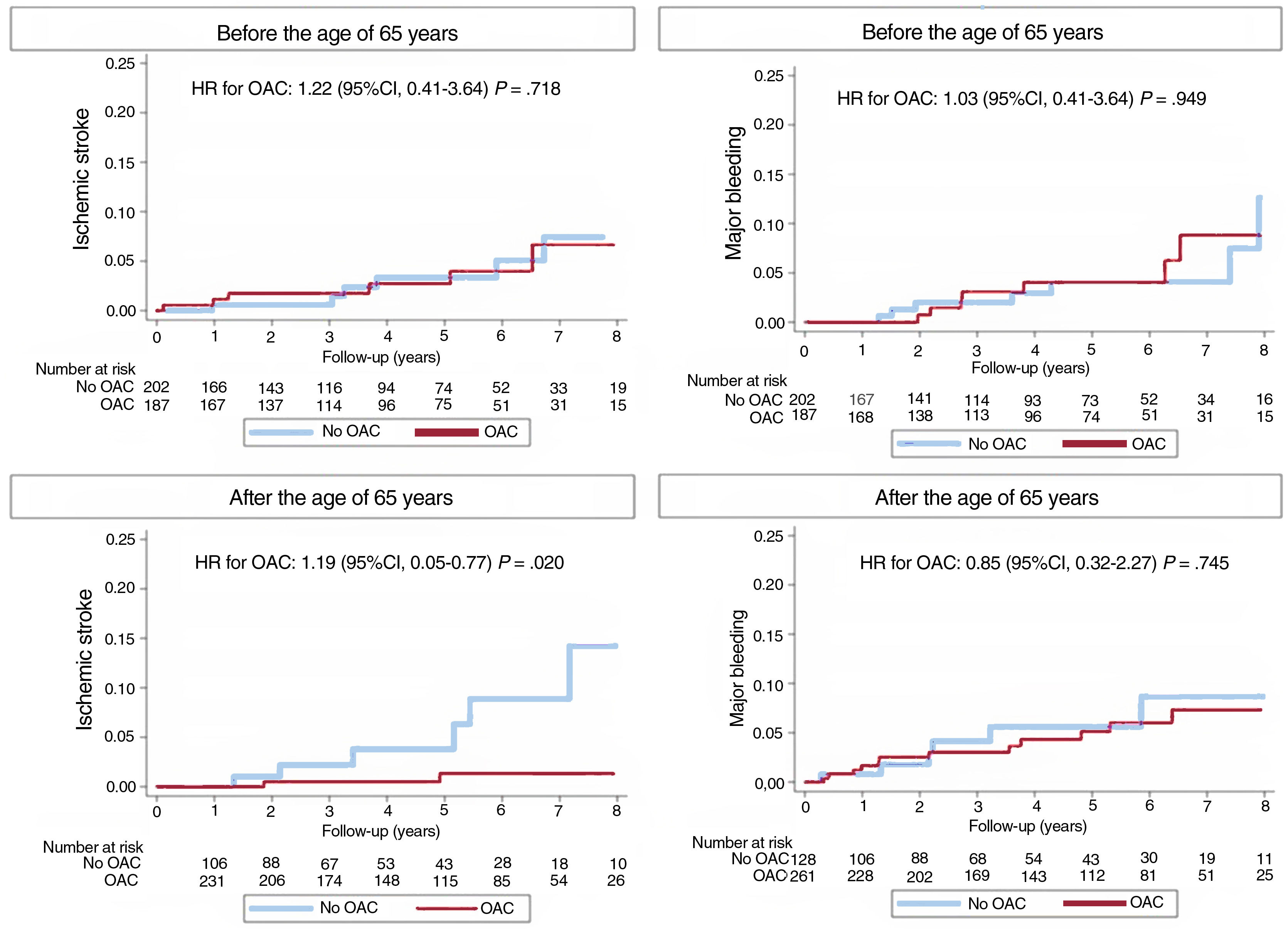
Before reaching the age of 65 years, 187 patients (48.1%) were receiving anticoagulation therapy. After reaching that age, this figure increased to 261 patients (67.1%), indicating a 19.0% increase in the anticoagulation rate at the age of 65 years. The analysis of patients younger than 65 years showed that oral anticoagulation did not lead to a lower risk of ischemic stroke or a higher risk of bleeding. However, at age 65 years, an increase in ischemic stroke was observed in non-anticoagulated patients compared with those receiving this therapy, while bleeding rates were similar (figure 1). Due to the relatively small sample size and limited number of episodes, subgroup analysis by sex was not feasible.
These findings, in which anticoagulation increased by only 19% in AF patients with a CHA2DS2-VASc score of 0 or 1 upon reaching the age of 65 years, indicate that up to 32.9% of patients are not provided this therapy (approximately 1 in every 3 patients). This has meaningful prognostic implications: anticoagulation in patients reaching 65 years was associated with a significant reduction in the ischemic stroke rate, with no increase in the bleeding risk. These data suggest that there is a need for systematic reassessment of the indication for anticoagulation in these patients, either by considering initiation of this therapy around age 65 years or, ideally, scheduling follow-up visits at the time patients reach that age.
FUNDINGNone.
ETHICAL CONSIDERATIONSWe accept the responsibility outlined by the International Committee of Medical Journal Editors. The study has been approved by the local ethics committee, which, given the retrospective and anonymized nature of the research, has waived the need for consent. Potential biases related to sex and gender have been taken into account.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence was not used.
AUTHORS’ CONTRIBUTIONSS. Raposeiras-Roubín, E. Abu-Assi, and A. Íñiguez-Romo designed the study; S. Raposeiras-Rubín and D. González-Fernández wrote the manuscript; D. González-Fernández, A. González-García, and C. Iglesias-Otero collected the data. All authors reviewed and approved the final draft.
CONFLICTS OF INTERESTS. Raposeiras-Roubín has received presentation fees from the following companies: Amgen, Abbott, Sanofi, Novartis, AstraZeneca, Daichii, Pfyzer-BMS, Bayer, and Boehringer. The remaining authors declare no conflicts of interest.