Alcohol septal ablation (ASA) aims to reduce the subaortic gradient in patients with symptomatic hypertrophic obstructive cardiomyopathy using alcohol to induce septal necrosis.1 Our aim was to describe the usefulness of 3-dimensional (3D) transthoracic echocardiography (TTE) with contrast in the localization and quantification of the extent of septal artery-dependent territory.
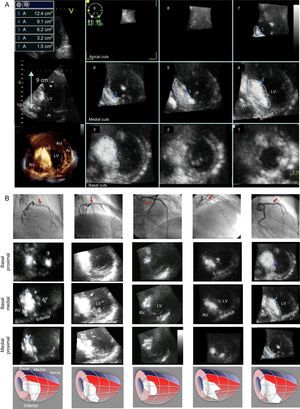
From 2012 onward, we prospectively enrolled 6 patients who then underwent ASA2 (Table). One patient was excluded due to poor imaging quality. Prior to ASA, 2D and 3D TTE were performed (Vivid E9, G.E. Healthcare). Following balloon occlusion of the selected septal artery, 1 to 2mL of angiographic contrast were injected, and 2D and 3D TTE were repeated. The selected septal artery-dependent region showed an increase in echographic signal (enhancement). After confirming the suitability of the septal artery, approximately 2mL of alcohol were injected. A multislice short-axis apicobasal view on TTE was used to trace contrast distribution and calculate the volume of septal artery-dependent myocardium (Figure A). For each slice, the area of enhanced tissue was multiplied by slice thickness, and the sum of these products gave the volume of enhanced myocardium. This volume was multiplied by myocardial density (1.05g/mL) to give the mass of enhanced myocardium. Immediate outcome and troponin I concentration at 24hours were recorded.
Variables of the 5 Patients Analyzed
| Patient number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Baseline information | |||||
| Age, years/ sex | 65/female | 73/female | 72/female | 85/female | 61/female |
| NYHA functional class | III | III | III | III | III-IV |
| Ejection fraction, % | 63 | 64 | 85 | 67 | 80 |
| Septal thickness, mm | 16 | 21 | 21 | 16 | 27 |
| LVOT gradient, mmHg | 71 | 140 | 60 | 44 | 110 |
| Mitral regurgitation, type | III | III | None | I | III-IV |
| Intraprocedural 3D TTE with contrast | |||||
| Contrast distribution in basal septum | Midline of anteroseptal and inferoseptal segments | Midline with inferoseptal spread | Midline with anteroseptal spread | Midline | Midline |
| Contrast distribution in midseptum | Midline with inferoseptal spread | Inferoseptal | Midline with anteroseptal spread | Midline | Inferoseptal |
| Contrast region corresponds to SAM? | Yes | Yes | Yes | Yes | Yes |
| Contrast localized distally? | No | No | No | No | No |
| Total ventricular mass, g | 200.1 | 246.5 | 318.5 | 144.3 | 196.0 |
| Contrast mass, g | 15.6 | 28.0 | 16.4 | 14.0 | 26.9 |
| Percentage of total ventricular mass, % | 7.8 | 11.4 | 5.2 | 9.7 | 13.8 |
| Intervention | |||||
| Septal artery | < 1.5 mm, medial, long | 2 mm, proximal, long | 1.7 mm, proximal | 1.8 mm, proximal, long | < 1.5 mm, proximal, short |
| Alcohol, mL | 2.0 | 2.0 | 2.5 | 2.0 | 3.0 |
| Baseline gradient, mmHg | 97 | 110 | 65 | 60 | 157 |
| Gradient post-ASA/on discharge, mmHg | 0/0 | 0/70 | 0/0 | 0/18 with Valsalva | 51/63 |
| Mitral regurgitation post-ASA/on discharge | Minimal/I | Minimal/I | None/None | None/I | II-III/I-II |
| Complications | None | None | None | PPM | None |
| Troponin I at 24h, ng/mL | 33.7 | 36.9 | 18 | 33 | 89 |
3D, 3 -dimensional; AA, alcohol ablation; ASA, alcohol septal ablation; LVOT, left ventricular outflow tract; NYHA, New York Heart Association; PPM, permanent pacemaker; SAM, systolic anterior motion; TTE, transthoracic echocardiography.
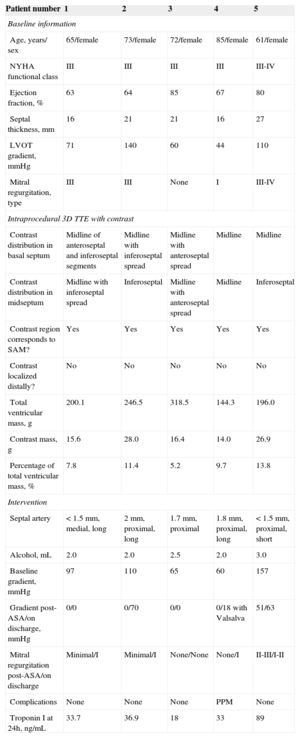
A: Three-dimensional multislice transthoracic echocardiography with contrast showing study of selected septal artery-dependent ventricular volume. Enhancement was distributed at the level of the basal septum and involved the inferoseptal and anteroseptal segments, extending to the midinferior septal segment. Calculated enhanced mass was 26.9g, 13.8% of total myocardial mass. B: Graphic representation of the 5 patients: top, angiography with selected septal arteries (arrow); center, multislice 3-dimensional transthoracic echocardiography study with contrast showing the enhancement distribution in 3 basal and medial planes; bottom, representative diagram of contrast distribution in each patient (septal artery region in red and enhancement in white). LV, left ventricle; RV, right ventricle.
In all 5 patients, enhancement was localized around the basal septum, with extension-to a greater or lesser degree-toward the superior or inferior septum. Extension toward the midline of the segments varied. Enhancement remained localized to the septum in 2 patients and tended to spread toward the inferior segment in the remaining 3. In all patients, enhancement was confirmed in the area of mitral valve contact with the septum. There was no spread to distal territories (Figure B). The septal artery-dependent myocardial mass was 20.21g (range, 14.03 g-28.00g), corresponding to 9.56% (5.2%-13.8%) of total myocardial mass. Troponin I concentration was 42.1 ± 27.2 ng/mL (range, 18-89 ng/mL).
In most patients, 3D TTE with contrast allowed precise localization and quantification of myocardial tissue dependent on the selected septal artery. It also allowed immediate confirmation of the real extent of contrast, whereas with 2D TTE with contrast, we cannot always be certain that all segments have been analyzed. In our cases, septal artery myocardial distribution appeared similar to necrosis distribution on post-ASA cardiac magnetic resonance (CMR). This distribution was noted predominantly at the junction of the anterior and inferior septa in the basal left ventricle and extended to the inferior portion of the midventricular septum.2 Without the availability of CMR after ASA, this concordance could not have been validated. Furthermore, 3D TTE with contrast allowed quantification of the septal artery-dependent myocardial mass in a similar way to CMR study of necrotic mass. The value obtained for contrast mass (15.6 g-33.6g) was similar to the necrotic mass values on post-ASA CMR published by Valeti et al3 (16g ±7g with 1.7mL ± 0.4mL of alcohol) and Yuan et al4 (27.9g ± 13.1g with 2.6mL ± 1.3mL). It is possible that the pre-ASA contrast study showed the entire septal artery-dependent vascular network, while post-ASA CMR showed only the necrotic area. There would undoubtedly have been dependent areas that did not necrose, and, in fact, as shown in the literature, necrosis also depends on the volume of alcohol injected.3 Using 3D TTE with contrast, information can be obtained similar to that from CMR, but prior to ASA, allowing selection of the most appropriate septal artery branch and prediction of the subsequent infarct area. In addition, CMR is unsuitable for patients with pacemakers.
Three-dimensional TTE requires a machine with a 3D transthoracic probe. Image acquisition and intraprocedural analysis of the localization and extent of contrast with 3D TTE did not take longer than with 2D TTE. Quantification of the septal artery-dependent mass was done manually on an external work station and took approximately 15 to 20minutes. Although this can be done during the procedure, it would be desirable to have a specific quantification program that reduced estimation time.
The main limitation of this study was the small sample size, due to the infrequency of ASA. This prevented us from establishing correlations between mass and enhancement and other variables.
In this study, we demonstrate the ease and usefulness of 3D TTE with contrast during ASA, as it allowed precise estimation of the target septal artery distribution and its dependent myocardial tissue size.
Further studies are needed to evaluate the potential usefulness of 3D TTE with contrast in selecting the target septal artery in complex cases with various possible branches, and in determining alcohol volume according to the dependent myocardial mass, which could reduce the need for permanent pacemakers.