Patients with congenital heart disease involving the right ventricular outflow tract (RVOT) sometimes require surgical or percutaneous treatment. This can lead to varying degrees of pulmonary insufficiency that, with time, can cause dilation and distortion of the right ventricle and the pulmonary trunk and branches, a condition known as dysfunctional RVOT.1 Before the advent of new self-expanding valve systems and dedicated devices, such as the Alterra Adaptive Prestent (Edwards Lifesciences, USA), many patients with dysfunctional RVOT were not suitable candidates for percutaneous pulmonary valve implantation (PPVI) because of large RVOTs and the absence of a stable landing zone for valve placement.1,2
The Alterra Adaptive Prestent is a self-expanding device designed to reconfigure the RVOT and enable PPVI with a 29-mm Edwards SAPIEN 3 transcatheter heart valve (THV) (figure 1A). Computer simulations of the sequential deployment of the Alterra device and the SAPIEN 3 THV have been described in the literature.3
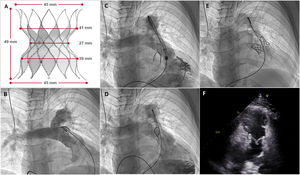
A: Alterra Adaptive Prestent. B, Initial pulmonary angiogram. C: partially deployed device. D: fully deployed device. E: Alterra device with the Edwards SAPIEN 3 valve. F: 2-dimensional echocardiography; parasternal short axis view showing the percutaneous valve inside the Alterra device.
We present the first 2 cases in Europe of the combined use of the Alterra device and the SAPIEN 3 THV deployed using the new Edwards pulmonic delivery system. On the eve of the procedures, potential deployment sites were assessed using 3-dimensional (3D) computed tomography (CT) images of the RVOTs. Physical patient-specific 3D models were also constructed to simulate deployment of the Alterra device. In both cases, prior informed consent was obtained for the compassionate use of the device and publication of the case reports.
The first patient was a 17-year-old boy with tetralogy of Fallot who had undergone transannular patch repair at 7 months of life but developed severe pulmonary insufficiency during follow-up. Magnetic resonance imaging (MRI) showed a right ventricular end-diastolic volume (RVTV) of 169mL/m2. A distal location was selected for the implantation. The minimum trunk diameter was 34mm (figure 1B, video 1 of the supplementary data). An initial attempt to deploy the Alterra device through the right pulmonary artery was unsuccessful due to a significant lack of coaxiality (figure 1C, video 2 of the supplementary data). A subsequent attempt via the left artery was successful (figure 1D, video 3 of the supplementary data). The Edwards pulmonic delivery system was then used to implant a 29-mm SAPIEN 3 THV (figure 1E, video 4 of the supplementary data). Echocardiographic assessment at 24hours showed correct valve implantation and no evidence of pulmonary insufficiency (figure 1F, videos 5 and 6 of the supplementary data).
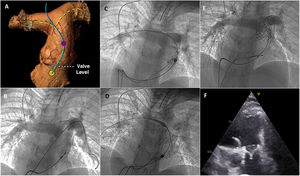
The second patient was a 16-year-old boy with congenital pulmonary stenosis treated by percutaneous valvuloplasty in the neonatal period; he had developed severe pulmonary insufficiency during follow-up. When he was 14 years old, he underwent percutaneous foramen ovale closure due to a history of cerebrovascular disease. At that time, MRI showed a minimum pulmonary trunk diameter of 30mm and an RVTV of 152mL/m2. Use of the Alterra Adaptive Prestent was considered when the boy was 16 years old, as he was showing diminishing physical capacity. Proximal deployment was simulated using 3D-CT angiography (figure 2A). The initial angiogram showed marked RVOT dilation (figure 2B, video 7 of the additional material). After an unsuccessful attempt to deploy the Alterra device through the left pulmonary artery due to a lack of coaxiality, it was decided to switch to the right artery (figures 2C and D, videos 8 and 9 of the supplementary data). PPVI of the 29-mm SAPIEN 3 THV using the pulmonic delivery system was completed without difficulty (figure 2E, video 10 of the supplementary data). Echocardiographic assessment at 24hours showed correct valve implantation and functioning (figure 2F, videos 11 and 12 of the supplementary data).
A: 3-dimensional computed tomography angiogram; simulated deployment of the Alterra Adaptive Prestent. B: initial pulmonary angiogram. C: partially deployed device. D: fully deployed device. E: Alterra device with the Edwards SAPIEN 3 valve. F: 2-dimensional echocardiography; parasternal short axis view showing the percutaneous valve inside the Alterra device.
The Alterra Adaptive Prestent is a self-expanding nitinol stent that reconfigures the RVOT to enable implantation of a 29-mm SAPIEN 3 THV. Its 27-mm central section provides a stable landing zone for the valve. It is partially covered with polytetrafluoroethylene, which seals the trunk, preventing perivalvular leaks and leaving the distal cells uncovered to facilitate blood flow to the pulmonary arteries. The device measures 45mm at both ends, which have flared tips to facilitate adaptation and anchorage when working with large, elastic RVOTs. It is fitted with an adjustment wheel for precise stent positioning and retraction even when 65% is uncovered.2
Although long-term follow-up data are not yet available (the first implants were performed some 5 years ago in the United States), Shahanavaz et al.1 reported 100% implantation success and no cases of valve dysfunction after 6 months in a series of 15 patients with a median age of 20 years and pulmonary trunk diameters ranging from 27 to 38mm.
Other self-expanding stents are available for the treatment of pulmonary insufficiency in patients with a dysfunctional RVOT, but unlike the Alterra device, they have an integrated valve. Examples are the Venus P-Valve (MedTech, China), the Pulsta transcatheter pulmonary valve (TaeWoong Medical, South Korea) and the Harmony TPV 22 and TPV 25 (Medtronic, USA).1,4 The Alterra device also differs in that it is available in a single size and is also shorter, enabling it to be implanted in the pulmonary trunk without invading the ventricular cavity. Edwards valves have been implanted in the pulmonary position since 2006, providing sufficient experience to help predict outcomes.5 This positioning also enables future valve-in-valve implantation, prolonging freedom from reintervention according to a retrospective European multicenter study involving hospitals that voluntarily collect data on procedures and patients treated with the SAPIENS 2 THV for PPV for reporting purposes (Mid-Term Outcomes of Transcatheter Pulmonary SAPIEN 3 Valve Implantation: An International Multicenter Registry).
The Alterra Adaptive Prestent represents a potential turning point in the treatment of dysfunctional RVOTs. By enabling PPVI, it could provide large numbers of patients with a viable alternative to surgical intervention.
FundingNone.
Authors’ ContributionsM. Figueras-Coll: study conception and design; data acquisition; data analysis and interpretation; writing of article; critical review of intellectual content; final approval. P. Betrián-Blasco: data acquisition; data analysis and interpretation; writing of article; critical review of intellectual content; final approval.
Conflicts of InterestNone.
Supplementary data associated with this article can be found in the online version available at doi:10.1016/j.rec.2023.08.001.