Pediatric inflammatory multisystem syndrome (PIMS) is a recently described entity with onset 2 to 6 weeks after a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children.1
We describe a 17-year-old girl with PIMS and severe myocardial involvement who presented to the emergency department (informed consent for this publication was given by the patient and her legal guardian). The patient had no relevant medical history, except for paucisymptomatic SARS-CoV-2 infection 1 month earlier that was treated on an outpatient basis. The patient reported headache, fever of up to 39°C, odynophagia, diffuse abdominal pain, vomiting, and dry cough lasting 1 week.
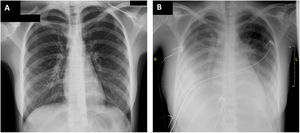
On arrival at the emergency department, blood pressure was 105/67mmHg, heart rate 120 bpm, baseline oxygen saturation 94%, and temperature 37.4°C. The physical examination revealed diffuse abdominal pain on palpation and skin rash in the hypogastric area. Electrocardiography and chest radiography on admission showed no abnormalities (figure 1A). Laboratory tests revealed elevated C-reactive protein (256 mg/L), D-dimer (4844 ng/mL), procalcitonin (4.04 ng/mL), high-sensitivity troponin I (162 ng/L), and N-terminal pro-brain natriuretic peptide (NT-proBNP) (9140 ng/L). Echocardiography showed preserved biventricular function with no other pathologic findings (). Abdominal and pelvic ultrasound and computed tomography ruled out acute abdominal disease, but revealed enlarged mesenteric lymph nodes smaller than 1cm. A gynecological ultrasound disclosed free fluid in the pouch of Douglas; a sample was collected under ultrasound guidance and showed a predominance of polymorphonuclear cells (85%) but cultures were negative. Blood cultures were also negative. Empirical antibiotic therapy was started with meropenem, clindamycin, and vancomycin, and the patient was admitted to the ward.
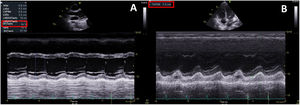
During the first 24hours of hospitalization, the patient exhibited poor clinical progress with hemodynamic instability, congestive heart failure with secondary respiratory failure, and cardiogenic shock and, therefore, she was transferred to the intensive care unit (ICU) (figure 1B). Bloodwork showed persistent elevation of C-reactive protein (278mg/L) and NT-proBNP (14 484 ng/L) levels. Troponin levels were trending down. Echocardiography was repeated, revealing severe biventricular dysfunction (left ventricular ejection fraction 35%; TAPSE, 12 mm) due to global hypocontractility, mild pulmonary hypertension, and bilateral pleural effusion but no pericardial effusion (figure 2, ). Vasoactive support was started with norepinephrine and dobutamine, as well as anticoagulant therapy at therapeutic doses (enoxaparin 60 mg/12 h). Because the patient met the diagnostic criteria for PIMS,1 she was given intravenous immunoglobulins at 2 g/kg and corticosteroid therapy 2 mg/kg/d for 5 days, leading to a drop in acute-phase reactants and clinical improvement. Vasoactive support was withdrawn after 5 days. Follow-up echocardiography showed complete recovery of biventricular function. Heart failure was resolved with intravenous diuretics and oxygen therapy through a low-flow nasal cannula. Antibiotic therapy was maintained during the patient's ICU stay.
Serology testing to screen for potential causes ruled out active infection due to Epstein-Barr virus, Coxiella burnetii, hepatitis viruses A, B, and C, toxoplasmosis, Treponema pallidum, adenovirus, parvovirus B19, and human immunodeficiency virus. The rapid antigen test for Streptococcus pyogenes was negative. The immunologic study (complement, lupus anticoagulant, ANCA, IgA, IgM, IgG, rheumatoid factor, etc.) was normal. Reverse transcription-polymerase chain reaction (RT-PCR) for SARS-CoV-2 was negative, but IgG spike antibodies were positive.
Infection due to SARS-CoV-2 in the pediatric population tends to be mild and, therefore, PIMS is a rare complication.1 It can progress to severe forms such as cardiogenic shock.2 The most commonly affected age bracket is 7 to 10 years,1 although it occasionally affects older children who may be diagnosed as late as age 19 or 21 years.2,3
The origin appears to be a delayed post-SARS-CoV-2 infection immune response, which is a heightened inflammatory response occurring weeks after acute infection, when RT-PCR for SARS-CoV-2 is negative and serology is positive.4
Most patients usually experience persistent fever for 3 or more days along with a clinical picture of hypotension or shock, signs of myocardial dysfunction (elevated troponin or NT-proBNP), evidence of coagulopathy (elevated D-dimer), acute gastrointestinal disease, or a complete or incomplete form of Kawasaki disease (KD) (skin rash, bilateral nonpurulent conjunctivitis, or mucocutaneous inflammatory signs in the mouth, hands, and feet).1 In addition, bloodwork will reveal elevated inflammatory markers (C-reactive protein, procalcitonin, erythrocyte sedimentation rate).1
The differential diagnosis for PIMS includes septic shock, streptococcal or staphylococcal toxic shock syndrome, KD, and acute abdomen.2 In our patient, rapid hemodynamic deterioration and severe biventricular dysfunction were determining factors for diagnosing PIMS. Some publications mention coronavirus infection as a possible cause for KD.5 As reported earlier, PIMS is characterized by similar symptoms to those of KD,2,6 hence both diseases could be part of the spectrum of the same disease with a common origin in coronavirus infection.
A case series from February 2020 to March 2021 reported higher mortality between ages 10 and 16 years.3 However, our patient's progress was favorable, and she recovered full cardiac function after 5 days in the ICU.
In conclusion, familiarity with PIMS is important in our current epidemiologic context, in view of the broad spectrum of the signs and symptoms of the disease. The condition affects pediatric patients, but may also be observed in young adults. Early diagnosis is essential, given its potentially aggressive nature.
FUNDINGThis study received no specific grants from public, commercial, or nonprofit institutions.
AUTHORS’ CONTRIBUTIONSC. Jiménez Martínez, V.M. Heras Hernando, and P. Gaite de Vicente conceived the article and contributed to the preparation of the manuscript. M.V. Godoy Tundidor, D. Afonso Rivero, and A. Gabán Diez critically reviewed the manuscript.
CONFLICTS OF INTERESTNone.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2021.12.011