The recent Academic Research Consortium for High Bleeding Risk (ARC-HBR) proposal did not consider acute coronary syndrome (ACS), by consensus, a bleeding criterion per se despite being a high bleeding risk (HBR) scenario. We investigated the applicability of the ARC-HBR classification and criteria in ACS patients.
MethodsPatients with ACS undergoing coronary stenting between 2012 and 2018 at a tertiary hospital were retrospectively classified as being at HBR if they met ≥ 1 major or ≥ 2 minor ARC-HBR criteria. The primary endpoint was the 1-year cumulative incidence of Bleeding Academic Research Consortium (BARC) 3 or 5 bleeding.
ResultsAmong 4412 patients, 29.5% were at HBR. The incidence of bleeding was higher in the HBR group than in the non-HBR group (9.4% vs 1.3%; P < .01). The rates of in-hospital periprocedural and postdischarge bleeding were also higher in the HBR group (4.3% vs 0.5% and 5.3% vs 0.9%, respectively; P < .01). Bleeding risk gradually increased with increasing ARC-HBR criteria: 1.8%, 5.0%, 9.4%, 16.8%, 25.2%, and 25.9% for 1 isolated minor criterion, ≥ 2 isolated minor criteria, 1 major criterion (isolated or plus 1 minor criterion), 1 major plus ≥ 2 minor criteria, ≥ 2 major criteria (isolated or plus 1 minor criterion), and ≥ 2 major plus ≥ 2 minor criteria, respectively. Sixteen (80%) out of 20 ARC-HBR criteria satisfied the ARC-HBR predefined cutoffs for BARC 3 or 5 bleeding risk.
ConclusionsThis study supports the use of the ARC-HBR classification and criteria in the ACS setting. The ARC-HBR classification provides an accurate major bleeding risk estimate and it seems suitable for the identification and management of patients at HBR.
Keywords
Bleeding represents a critical safety endpoint in acute coronary syndrome (ACS) patients.1–6 Accurate identification of ACS patients who are at high bleeding risk (HBR) is essential to maximize the benefits of antithrombotic therapy and invasive procedures while minimizing bleeding events.1,2,6–9 Until recently, there has been no consensus on the definition of HBR in patients undergoing percutaneous coronary intervention (PCI). In May 2019, the Academic Research Consortium for High Bleeding Risk (ARC-HBR) aimed to standardize the definition and indicative criteria of HBR.3 Specifically, HBR was defined as a Bleeding Academic Research Consortium (BARC)4 type 3 or 5 bleeding risk of ≥ 4% at 1 year or risk of an intracranial hemorrhage (ICH) of ≥ 1% at 1 year. The ARC-HBR consensus identified 20 major and minor clinical and analytical criteria and proposed considering patients as being at HBR if they met at least 1 major or 2 minor criteria.3
The ARC-HBR scope was the overall PCI population and, by consensus, ACS was not considered a ARC-HBR criterion per se. However, ACS patients are usually prescribed more intensive and longer-duration dual antiplatelet therapy (DAPT), and have substantially different clinical and procedural characteristics than patients undergoing elective PCI for stable coronary artery disease.1,2,5,9–13 These aspects are likely to impact the ARC-HBR predefined cutoffs for bleeding and the applicability of the ARC-HBR criteria in the particular ACS scenario.
Accordingly, in this study of unselected ACS patients, we investigated the applicability of the ARC-HBR proposal in the ACS setting and compared the performance of the new ARC-HBR classification with the PRECISE-DAPT classification system14 in predicting major bleeding events.
METHODSPatient populationThis was a retrospective observational study based on the CardioCHUVI (Cardiología del Complejo Hospitalario Universitario de VIgo) ACS registry (NCT03664388), in which the study participants were all patients with a primary and final diagnosis of ACS consecutively admitted to our department between January 2012 and September 2018. Secondary acute coronary syndromes during a hospital admission for another cause were not included in the registry. Demographic, clinical and angiographic variables, as well as those relating to management and complications were gathered prospectively by the department's cardiologists. Patients were treated according to their main physician's criteria.
All patients were included in administrative databases of the regional electronic medical records system at both hospital and outpatient level. This system allowed recording of clinical and laboratory data, pharmacological treatment, bleeding events, and vital status. Electronic medical records were meticulously reviewed by 2 independent clinicians at the adjudication of events of interest.
For this study, we comprehensively reviewed the variables related to the ARC-HBR criteria and retrospectively retrieved data on some ARC-HBR criteria which were not initially available in our database (). This approach enabled us to apply the 20 ARC-HBR criteria (14 major and 6 minor) as originally described,11 except for the minor criterion “oral nonsteroidal anti-inflammatory drugs or steroid use”, which was defined as chronic instead of an expected daily intake for ≥ 4 weeks.11
The initial cohort of the present study comprised 5959 patients. For this study, patients were excluded for the following reasons: a) not undergoing in-hospital coronary stenting (1436 patients); b) not receiving DAPT (69 patients); c) missing data on the ARC-HBR criteria (18 patients) or for calculating the PRECISE-DAPT score (11 patients); and d) unavailable data on bleeding or vital status (13 patients). Heparin was used for procedural anticoagulation and periprocedural use of glycoprotein IIb/IIIa inhibitors was left to the discretion of the operator. DAPT duration was determined at the discretion of the treating clinicians.
The study conformed to the principles outlined in the Declaration of Helsinki, and was approved by the local ethics committee (Galicia; code HAC-2017-05, registry 2017/290).
Endpoints, definition, and follow-upThe primary endpoints were the 1-year incidences of first BARC 3 or 5 bleeding and first ICH. Bleeding outcomes included peri-PCI, in-hospital bleeding and postdischarge bleeding. The secondary endpoint was the 1-year incidence of the composite of cardiovascular death, myocardial infarction, or ischemic stroke.
Cardiovascular death and myocardial infarction were defined according to Hicks et al.15 and the Third Universal Definition16), respectively. Diagnosis of ischemic stroke required an acute neurological deficit lasting ≥ 24hours with at least 1 imaging test and neurologist confirmation.
Patients were classified as being at HBR according to the ARC-HBR classification in the presence of ≥ 1 major criterion or ≥ 2 minor criteria. Since the 5-item (hemoglobin, age, leucocytes, serum creatine clearance, and previous bleeding) PRECISE-DAPT score is currently the recommended system (class IIb) for HBR classification by the European Society of Cardiology,2 we used the PRECISE-DAPT HBR classification (≥ 25 vs < 25 points) as a comparator to assess the relative performance of the new ARC-HBR classification.
The ascertainment of endpoints was performed by 2 trained independent cardiologists and was carried out between December and February 2020.
Statistical methodsQuantitative data are presented as mean ± SD or median and interquartile range [IQR], as appropriate. Categorical variables are presented as numbers (%). Qualitative variables were compared by the chi-square test or Fisher exact test; quantitative variables were compared by the Student t-test or Mann-Whitney test. Both ARC and PRECISE-DAPT classifications were entered into separate Fine-Gray competing risk models (with death unrelated to bleeding as a competing risk) to test their association with the primary endpoints. The ability of each classification to separate HBR patients from non-HBR patients was checked by cumulative incidence curves and compared using the Fine-Gray test. The discrimination ability of the 2 classifications for predicting bleeding events was estimated via the c-statistic.
To evaluate the prognostic value of the ARC-HBR criteria, we generated and plotted the cumulative incidence for the primary endpoint by the number of the ARC-HBR criteria and examined if each ARC-HBR major or minor criterion, in isolation, met the ARC predefined cutoff for BARC 3 or 5 bleeding risk. Finally, the risk of the secondary endpoint by groups of HBR and non-HBR of ARC was assessed using the Fine-Gray method (with noncardiovascular death as a competing event). The c-statistic, as a discrimination measure at predicting the ischemic event with ARC-HBR, was also calculated.
All analyses were crudes since both the ARC-HBR binary classification and its individual criteria were established to be used without any statistical adjustments. The association between the HBR classifications with outcomes was expressed as subhazard ratios (sHR) with their 95% confidence intervals (95%CI). The bootstrap method (500 iterations) was used to estimate statistical significance, subhazard ratio (sHR), and c-statistic values as well as 95%CIs. Statistical analyses were 2-tailed and were mainly performed using STATA/MP 15.1. R 3.6.3 was used for calculating the c-statistic values via the “pec” package. Statistical significance was set at P < .05.
RESULTSBaseline characteristics by HBR statusThe present study cohort comprised 4412 ACS patients treated with DAPT and coronary stenting. Table 1 summarizes the differences in clinical and procedural characteristics between patient groups according to the ARC-HBR-classified HBR status.
Patient baseline characteristics and medications
| Entire cohortN = 4412 | HBRn = 1303 | Non-HBRn = 3109 | P | |
|---|---|---|---|---|
| PRECISE-DAPT ≥ 25 points | 1774 (40.2%) | 1076 (82.6) | 698 (22.5) | < .001 |
| Age, y | 59.6 ± 11.2 | 75.8 ± 10.2 | 58.0 ± 11.1 | < .001 |
| Females, % | 986 (22.3) | 539 (41.4) | 447 (14.4) | < .001 |
| Body mass index, kg/m2 | 28.1 ± 4.7 | 28.1 ± 4.2 | 29.3±4.4 | < .001 |
| Current smoking, % | 1699 (38.5) | 192 (14.7) | 1507 (48.5) | < .001 |
| Hypertension, % | 2731 (61.9) | 1039 (79.7) | 1692 (54.4) | < .001 |
| Diabetes mellitus, % | 1081 (24.5) | 468 (35.9) | 613 (19.7) | < .001 |
| Prior coronary artery disease, % | 1072 (24.3) | 414 (31.8) | 658 (21.2) | < .001 |
| Prior atrial fibrillation, % | 327 (7.4) | 249 (19.1) | 78 (2.5) | < .001 |
| Prior congestive heart failure, % | 152 (3.4) | 89 (6.8) | 63 (2.0) | < .001 |
| Peripheral artery disease, % | 381 (8.6) | 183 (14.0) | 198 (6.4) | < .001 |
| Chronic obstructive pulmonary disease, % | 434 (9.8) | 202 (15.5) | 232 (7.5) | < .001 |
| Systemic immune-mediated diseases, % | 119 (2.7) | 43 (3.3) | 76 (2.4) | .12 |
| Chronic arthropathy, % | 388 (8.8) | 213 (16.3) | 175 (5.6) | < .001 |
| Psychiatric disorder, % | 239 (5.4) | 80 (6.1) | 159 (5.1) | .17 |
| Type of acute coronary syndrome | < .001 | |||
| STEMI, % | 2354 (53.4) | 592 (45.4) | 1762 (56.7) | |
| NSTEACS, % | 2058 (46.3) | 711 (54.6) | 1347 (43.3) | |
| Killip ≥ 2 or LVEF ≤ 30%, % | 714 (16.2) | 343 (26.3) | 371 (11.9) | < .001 |
| Hemoglobin, g/dL | 14.2 ± 1.8 | 12.9 ± 2.0 | 14.8 ± 1.4 | < .001 |
| eGFR, mL/min/1.73 m2 | 106.7 ± 23.5 | 93.4 ± 31.0 | 112.3 ± 16.5 | < .001 |
| Leucocyte count, 103/L | 10 137 ± 4191 | 10 460 ± 4247 | 9313 ± 4013 | < .001 |
| Transradial approach, % | 4161 (94.3) | 1184 (90.9) | 2977 (95.8) | < .001 |
| DES, % | 2616 (59.3) | 629 (48.3) | 1987 (63.9) | < .001 |
| Glycoprotein IIb/IIIa inhibitors, % | 490 (11.1) | 99 (7.6) | 391 (12.6) | < .001 |
| Multivessel disease, % | 1932 (43.8) | 637 (48.9) | 1295 (41.7) | < .001 |
| Lesion location, % | ||||
| Left main | 79 (1.8) | 39 (3.0) | 40 (1.3) | < .001 |
| Left anterior descending | 1482 (33.6) | 512 (39.3) | 970 (31.2) | < .001 |
| Left circumflex | 1046 (23.7) | 344 (26.4) | 702 (22.6) | .01 |
| Right coronary | 1209 (27.4) | 377 (28.9) | 832 (26.8) | .17 |
| Saphenous vein graft | 40 (0.9) | 24 (1.8) | 16 (0.5) | < .001 |
| Complete revascularization, % | 2237 (50.7) | 572 (43.9) | 1665 (53.6) | < .001 |
| Total number of stents | 1.8 ± 1 | 1.8 ± 1.1 | 1.7 ± 1.0 | .26 |
| Total stent length, mm | 34 ± 23 | 33 ± 22 | 34 ± 24 | .27 |
| Stent diameter < 3.0mm, % | 1730 (39.2) | 577 (44.3) | 1153 (37.1) | < .001 |
| Aspirin, % | 4412 (100) | 1303 (100) | 3109 (100) | - |
| Clopidogrel, % | 3587 (81.3) | 1196 (91.8) | 2391 (76.9) | < .001 |
| Ticagrelor or prasugrel, % | 825 (18.7) | 107 (8.2) | 718 (23.1) | < .001 |
| OAC at discharge, % | 205 (4.6) | 205 (15.7) | 0 (0) | < .001 |
| DAPT duration, mo | 12.1[7.2-12.0] | 10.2[1.2-12.0] | 12.0[11.4-12.0] | < .001 |
| DAPT plus OAC duration, mo | 1.9[1.0-6.9] | 1.9[1.0-6.9] | - | - |
| Statin, % | 4266 (96.7) | 1235 (94.8) | 3031 (97.5) | < .001 |
| Beta-blocker, % | 3600 (81.6) | 992 (76.1) | 2608 (83.9) | < .001 |
| ACEinhibitor/ARB-II, % | 2965 (67.2) | 879 (67.5) | 2086 (67.1) | .78 |
ACE inhibitor, angiotensin conversing enzyme inhibitor; ARB-II, angiotensin II receptor antagonist; DAPT, dual antiplatelet therapy; DES, drug eluting stent; eGFR, estimated glomerular filtration rate; HBR, high bleeding risk; LVEF, left ventricular ejection fraction; NSTEACS, non–ST-elevation acute coronary syndrome; OAC, oral anticoagulation; STEMI, ST-segment elevation myocardial infarction.
The data are presented as No. (%), mean ± standard deviation, or median [range].
There were 1303 patients (29.5%) in the ARC-HBR group and 3109 in the ARC non-HBR group. More patients were classified as being at HBR by PRECISE-DAPT (n = 1,774; 40.2%) (McNemar test P-value < .001).
Compared with the ARC non-HBR group, patients in the ARC-HBR group were older, had a higher prevalence of women and cardiovascular as well as noncardiovascular comorbidities. They also had less frequent use of the transradial approach, a higher proportion of multivessel disease, and less often received ticagrelor or prasugrel. Median DAPT duration was shorter in the ARC-HBR group (10.2 months) than in the ARC non-HBR group (12.0 months); P < .001 (table 1).
Prevalence of the ARC-HBR criteriaTable 2 shows the prevalence of the ARC-HBR criteria. The most common major criteria were hemoglobin < 11g/dL (5.0%), oral anticoagulant use (4.6%), spontaneous bleeding (1.5%), active malignancy (1.3%), and estimated glomerular filtration rate < 30mL/min/1.73 m2 (1.3%). The remaining 11 major criteria were rarely observed (≤ 0.5%).
Prevalence, in decreasing order, of major and minor criteria of the Academic Research Consortium for High Bleeding Risk (ARC-HBR)
| Entire cohortN = 4412 | HBRn = 1303 | Non-HBRn = 3109 | P | |
|---|---|---|---|---|
| ARC-HBR major criteria | ||||
| Hemoglobin < 11 g/dL | 221 (5.0) | 221 (17.0) | 0 (0) | < .001 |
| Anticipated use of long-term OAC | 205 (4.6) | 205 (15.7) | 0 (0) | < .001 |
| Spontaneous bleeding requiring hospitalization or transfusion within 6 mo before index PCI or at any time, if recurrent | 64 (1.5) | 64 (4.9) | 0 (0) | < .001 |
| Active malignancy (excluding nonmelanoma skin cancer) within 12 mo before index PCI | 59 (1.3) | 59 (4.5) | 0 (0) | < .001 |
| eGFR < 30 mL/min/1.73 m2 | 57 (1.3) | 57 (4.4) | 0 (0) | < .001 |
| Previous spontaneous ICH at any time | 24 (0.5) | 24 (1.8) | 0 (0) | < .001 |
| Recent major surgery or major trauma within 30 days before index PCI | 24 (0.5) | 24 (1.8) | 0 (0) | < .001 |
| Liver cirrhosis with portal hypertension | 21 (0.5) | 21 (1.6) | 0 (0) | < .001 |
| Moderate-severe ischemic stroke within 6 mo before index PCI | 17 (0.4) | 17 (1.3) | 0 (0) | < .001 |
| Nondeferrable major surgery on DAPT | 15 (0.3) | 15 (1.2) | 0 (0) | < .001 |
| Platelet count < 100 × 109/L | 11 (0.3) | 11 (0.8) | 0 (0) | < .001 |
| Chronic bleeding diathesis | 9 (0.2) | 9 (0.7) | 0 (0) | < .001 |
| Previous traumatic ICH within 12 mo before index PCI | 4 (0.1) | 4 (0.3) | 0 (0) | .007 |
| Brain arteriovenous malformation, % | 3 (0.07) | 3 (0.2) | 0 (0) | .02 |
| ARC-HBR minor criteria | ||||
| Age ≥ 75 y | 1190 (27.0) | 906 (69.5) | 284 (9.1) | < .001 |
| eGFR 30-59 mL/min/1.73 m2 | 863 (19.6) | 697 (53.5) | 166 (5.3) | < .001 |
| Hemoglobin 11-12.9 g/dL for men and 11-11.9 g/dL for women | 472 (10.7) | 310 (23.8) | 162 (5.2) | < .001 |
| Chronic use of oral NSAIDs or steroids | 376 (8.5) | 209 (16.0) | 167 (5.4) | < .001 |
| Any ischemic stroke at any time not meeting the major criterion | 150 (3.4) | 106 (8.1) | 44 (1.4) | < .001 |
| Spontaneous bleeding requiring hospitalization or transfusion within 12 mo before index PCI, not meeting the major criterion | 15 (0.3) | 9 (0.7) | 6 (0.2) | .01 |
ARC-HBR, Academy Research Consortium for High Bleeding Risk; DAPT, dual antiplatelet therapy; eGFR, estimated glomerular filtration rate; ICH, intracranial hemorrhage; NSAIDs, nonsteroidal anti-inflammatory drugs; OAC, oral anticoagulation; PCI, percutaneous coronary intervention.
The data are presented as No. (%).
The most common minor criteria were age ≥ 75 years (27.0%), estimated glomerular filtration rate 30 to 59mL/min/1.73 m2 (19.6%), mild anemia (10.7%), oral nonsteroidal anti-inflammatory drug or steroid use (8.5%), and prior ischemic stroke (3.4%). Spontaneous bleeding was rarely observed (0.3%).
BARC type 3 or 5 bleeding at 1-yearThroughout 11.6 ± 1.7 months, 162 patients had a BARC 3 or 5 bleeding event; of them, 70 had periprocedural in-hospital bleeding.
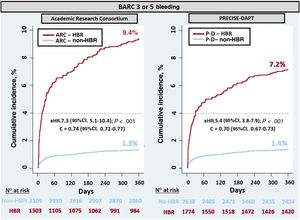
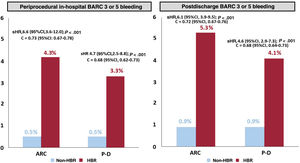
By ARC groups, the overall incidence of BARC 3 or 5 bleeding was significantly higher in the HBR group than in the non-HBR group (9.4% vs 1.3%; P < .001; sHR, 7.3; 95%CI, 5.1-10.4) (figure 1). The rate of periprocedural in-hospital bleeding was 4.3% in the HBR group (vs 0.5%, P < .001; sHR, 6.6; 95%CI, 3.6-12.0), whereas postdischarge bleeding rate was 5.3% (vs 0.9%, P < .001; sHR, 6.1; 95%CI, 3.9-9.5) (figure 2).
Cumulative incidence for BARC 3 or 5 bleeding by high bleeding risk groups according to the ARC-HBR and the PRECISE-DAPT classifications. 95%CI, 95% confidence interval; ARC-HBR, Academy Research Consortium for High Bleeding Risk; BARC, Bleeding Academy Research Consortium; C =, c-statistic; P-D, PRECISE-DAPT (Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy); sHR, subhazard ratio.
Cumulative incidence for periprocedural in-hospital and postdischarge bleeding by high bleeding risk groups according to the ARC-HBR and the PRECISE-DAPT classifications. 95%CI, 95% confidence interval; ARC, Academy Research Consortium; BARC, Bleeding Academy Research Consortium; C =, c-statistic; HBR, High Bleeding Risk; P-D, PRECISE-DAPT (Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy); sHR, subhazard ratio.
By PRECISE-DAPT groups, the overall incidence of BARC 3 or 5 bleeding was significantly higher in the HBR than in the non-HBR group (7.2% vs 1.4%; P < .001; sHR, 5.4; 95%CI, 3.8-7.9) (figure 1). The rate of periprocedural in-hospital bleeding was 3.3% in the HBR group (vs 0.5%; P < .001; sHR, 4.7; 95%CI, 2.5-8.8), while postdischarge bleeding rate was 4.1% (vs 0.9%; P < .001; sHR, 4.6; 95%CI, 2.9-7.3) (figure 2).
Both classifications were significantly associated with BARC 3 or 5 bleeding, albeit ARC showed a more pronounced association overall (sHR = 7.3 vs 5.4), with periprocedural in-hospital bleeding (sHR = 6.6 vs 4.7), and with postdischarge bleeding (6.1 vs 4.6). The c-statistic values also favored ARC overall (C = 0.74 vs 0.70), for periprocedural bleeding (0.73 vs 0.68), and for postdischarge bleeding 0.72 vs 0.68, respectively).
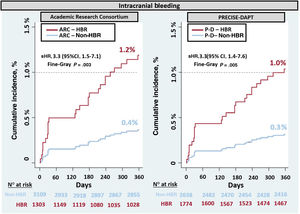
Intracranial hemorrhage at 1 yearICH occurred in 26 patients out of the total population. The incidence of ICH was significantly higher in the ARC-HBR group than in the non-HBR group (1.2% vs 0.4%; P = .003; sHR, 3.3; 95%CI, 1.5-7.1). The same pattern was observed using PRECISE-DAPT (1.0% vs 0.3%; P = .005; sHR, 3.3; 95%CI, 1.4-7.6) (figure 3).
Cumulative incidence for intracranial hemorrhage by high bleeding risk groups according to the ARC-HBR and the PRECISE-DAPT classification systems. 95%CI, 95% confidence interval; ARC, Academy Research Consortium; HBR, high bleeding risk; P-D, PRECISE-DAPT (Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy); sHR, subhazard ratio.
Both classification systems were significantly associated with ICH risk to a similar extent (sHR = 3.3 for both), and displayed similar c-statistics (C = 0.65).
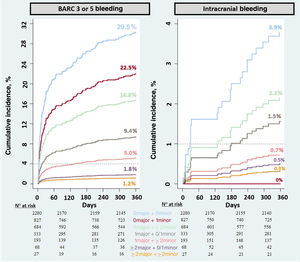
Prognostic value of the ARC-HBR criteriaThe risk of bleeding outcomes increased as the number of minor and major criteria was higher (figure 4): for BARC 3 or 5 bleeding, the risk was 1.8%, 5.0%, 9.4%, 16.8%, 22.5%, and 29.5% for 1 isolated minor criterion, ≥ 2 isolated minor criteria, 1 major criterion (isolated or plus 1 minor criterion), 1 major criterion plus ≥ 2 minor criteria, ≥ 2 major criteria (isolated or plus 1 minor criterion), and ≥ 2 major criteria plus ≥ 2 minor criteria, respectively.
For ICH, the risk was 0.3%, 0.5%, 0.7%, 1.5%, 2.2%, and 3.9% for 1 isolated minor criterion, ≥ 2 isolated minor criteria, 1 major criterion (isolated or plus 1 minor criterion), 1 major criterion plus ≥ 2 minor criteria, and ≥ 2 major criteria plus ≥ 2 minor criteria, respectively. No ICH events were observed in patients with 2 major criteria (isolated or plus 1 minor criterion).
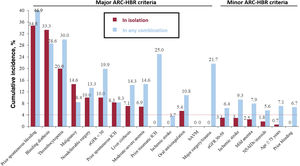
The 1-year rates of BARC 3 or 5 bleeding associated with each ARC-HBR criteria, in isolation, are shown in figure 5. Sixteen out of 20 ARC criteria (80%) met the predefined cutoffs. That is, among the 14 major criteria, 10 (71.4%) were associated, each in isolation, with a BARC 3 or 5 bleeding risk ≥ 4%. Prior traumatic ICH, recent major surgery/trauma, prior moderate-severe ischemic stroke, and brain arteriovenous malformation were not associated, in isolation, with a BARC 3 or 5 bleeding risk ≥ 4%. All 6 ARC minor criteria were associated, each in isolation, with a BARC 3 or 5 bleeding risk < 4%.
Cumulative incidence for BARC 3 or 5 bleeding at 1 year by each of the ARC-HBR criteria, in isolation*. ARC-HBR, Academy Research Consortium for High Bleeding Risk; BARC, Bleeding Academy Research Consortium; bAMV, brain arteriovenous malformation; eGFR, estimated glomerular filtration rate; ICH, intracranial hemorrhage; NSAIDs, nonsteroidal anti-inflammatory drugs.
*A complete description for each criterion is provided in table 2.
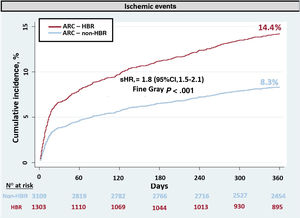
The ischemic endpoint was observed in 432 patients (329 myocardial infarctions, 56 ischemic strokes, and 47 cardiovascular deaths). The incidence of ischemic events was 2-fold higher in the ARC-HBR group than in the ARC non-HBR group (14.3% vs 8.3%; P < .001; sHR, 1.8; 95%CI, 1.5-2.1) (figure 6). The c-statistic for predicting the ischemic endpoint with ARC-HBR was 0.57 (95%CI, 0.55-0.59).
DISCUSSIONThe main findings of the present analysis are as follows: a) ARC-HBR classified a significantly lower proportion of patients as being at HBR (≈30%) than PRECISE-DAPT (≈40%); b) patients at HBR according to ARC-HBR and PRECISE-DAPT had high major bleeding risks fulfilling the ARC predefined thresholds; c) the ARC system exhibited attributes of a more effective classifier of HBR than did PRECISE-DAPT; d) there was an additive effect with bleeding risk gradually rising as the number of the ARC criteria increased; e) most (80%) ARC major criteria, in isolation, met the predefined threshold for BARC 3 or 5 bleeding risk; and f) HBR patients were also at significantly higher risk of ischemic events, but the predictiveness of ARC-HBR for these events was rather weak.
The ARC-HBR proposal3 is expected to help physicians to correctly identify HBR patients in order to optimally tailor the bleeding avoidance strategies in patients undergoing PCI. ACS, by consensus, was not considered a ARC-HBR criterion per se despite being a particular scenario with specific patient-related and periprocedural-related features predisposing for a higher bleeding risk than other stable PCI scenarios.5,9–13 These considerations may affect the validity of the ARC-HBR predefined cutoffs and criteria in the ACS context. Although the authors of the ARC-HBR state that the increased bleeding risk in patients with ACS is attributable to more aggressive antiplatelet therapy rather than to ACS per se, no data were reported in the ARC-HBR document to support such an approach. Moreover, previous studies found that ACS was an independent predictor of bleeding while in-hospital or at 30 days after PCI.5,12 Accordingly, more data are needed before recommending the use of the ARC-HBR definition and criteria in ACS patients.
Our data suggest that applying the ARC-HBR classification could correctly identify patients who were truly at HBR and, thus, provide support to the use of that classification in the ACS setting. Moreover, our findings validate most (80%) of the ARC-HBR criteria when fulfilled in isolation except for 4 major criteria. Importantly, the prevalence of these 4 criteria was ≤ 0.5% which could have limited their prognostic value.
The validity of ARC-HBR proposal was recently examined in all-comers PCI patients.17–19 They found that ARC-HBR definition successfully identified patients with significantly higher rates of bleeding events after PCI. Nevertheless, the results of previous studies should be interpreted in light of the missing or readapted definitions for several ARC-HBR criteria and the use of bleeding endpoints other than BARC 3 or 5 bleeding or ICH. Moreover, specific data on bleeding in ACS syndrome patients and periprocedural in-hospital bleeding as well as postdischarge bleeding in these patients were not addressed in these studies, since they only included a low percentage of patients with ACS (less than half of the total population). An important finding in our analysis was the 7-fold risk of periprocedural BARC 3 or 5 bleeding observed in the HBR group according to ARC-HBR in ACS patients. In comparison, studies that included all-comer PCI patients reported a 2- to 3-fold risk of bleeding in the HBR group. This is relevant in an ACS population in light of the use of more potent antithrombotic therapies and other procedural aspects that increase their bleeding risk compared with patients undergoing elective PCI. For instance, procedural (artery approach, vascular closure devices) and pharmacological (multiple antithrombotic agents) factors may become less relevant in predicting the risk of postdischarge bleeding, whereas patient characteristics and DAPT intensity and duration may become more important after discharge.
Patients at HBR were also at a higher burden of ischemic events than non-HBR patients. This finding may be explained by the fact that ischemic risk is closely related to bleeding risk, and antithrombotic as well as invasive therapies need to balance the benefit from prevention of ischemic events against serious bleeding risk. Of note, the ARC-HBR dichotomous classification was a weaker prognosticator for ischemic events (c = 0.57) than for BARC 3 or 5 bleeding risk (c = 0.74). This suggests that the ARC-HBR classification more closely correlates with bleeding risk than with ischemic risk. The prior finding is likely attributable to the fact that the ARC-HBR criteria is clearly oriented toward predicting bleeding with few shared criteria for ischemic events, such as age and renal dysfunction.
PRECISE-DAPT was used as a comparator in this study. It should be noted that PRECISE-DAPT was specifically designed to predict postdischarge bleeding but not in-hospital bleeding. However, the ARC-HBR system showed attributes of a better classifier of both in-hospital and postdischarge BARC 3 or 5 bleeding risk in HBR patients than did the PRECISE-DAPT system. This is not surprising since the 5-item PRECISE-DAPT was derived from a selected low-risk population and did not include other important bleeding prognosticators compared with the 20-item ARC-HBR system. Another advantage of the ARC-HBR system is that it allows patients to be classified in a relatively simple way, on the basis of fulfilling at least 1 major criterion or 2 minor criteria with no need for computation.
Bleeding avoidance strategies such as transradial access, targeted periprocedural anticoagulation, and the use of vascular closure devices in addition to shortening DAPT duration have been demonstrated to be safe and effective therapeutic options in HBR patients.6,8,9,20 The use of the ARC-HBR criteria-based classification system can accurately identify those patients with a high likelihood of major bleeding, which may help physicians select the most appropriate therapeutic strategy.
Strengths and limitationsThe major strengths of this study include the use of the ARC-HBR criteria as originally conceived (excepting oral nonsteroidal anti-inflammatory drugs/steroids use), the concomitant assessment of BARC 3 or 5 bleeding and ICH, and the independent assessment of outcomes. Important limitations, however, need to be addressed. This was a retrospective study with the limitations inherent to this type of design. The single-center design of this study may limit the generalizability of our findings, and the study power was limited to adequately exploring ICH by all individual ARC-HBR criteria.
The findings and conclusions of this study do not apply to the subgroup of patients treated without DAPT (n = 69), as they were excluded from the study to reduce the heterogeneity of the population studied and minimize possible biases.
CONCLUSIONSAmong ACS patients undergoing coronary stenting, approximately 30% are at HBR by ARC-HBR, substantially less than with PRECISE-DAPT (≈40%). The ARC-HBR classification provides more accurate risk estimates at predicting the risk of major bleeding and seems a more suitable clinical tool for the identification and management of HBR patients. The vast majority of ARC-HBR criteria, in isolation, are valid bleeding criteria in ACS patients undergoing coronary stenting mainly with the transradial arterial approach.
- -
Proper identification of acute coronary syndrome (ACS) patients with high bleeding risk is essential to optimize invasive and pharmacological treatment.
- -
Academic Research Consortium for High Bleeding Risk (ARC-HBR) has recently standardized the definition of high bleeding risk for patients undergoing percutaneous coronary interventions in a stable coronary artery disease setting.
- -
ACS patients are usually exposed to a greater bleeding risk, given the more aggressive treatment they receive and the underlying pathophysiological process itself.
- -
ARC-HBR has not considered the ACS a per se criterion, so the performance of ARC-HBR in this scenario is not clear.
- -
The ARC-HBR system showed adequate applicability to determine ACS patients at high bleeding risk.
- -
Application of the ARC-HBR criteria was more effective in identifying ACS patients at HBR than the Precise-DAPT score.
- -
ARC-HBR criteria demonstrated an additive effect in bleeding risk prediction: the risk gradually became higher as the number of the ARC criteria increased.
- -
Patients at HBR were also at significantly higher risk of ischemic events. However, the ability of ARC-HBR to predict these events was rather weak.
None.
AUTHORS’ CONTRIBUTIONSThe corresponding author is responsible for ensuring that the descriptions are accurate and agreed by all authors. E. Abu-Assi conceived the presented idea. All authors, except Andrés Íñiguez Romo, participated in data acquisition and curation. E. Abu-Assi established the methods and performed the analyses. S. Raposeiras-Roubín validated the research output. All authors discussed the results and commented on the manuscript. All authors revised the manuscript critically for important intellectual content, agreed for all aspects of the work, and approved the final version to be published.
CONFLICTS OF INTERESTE. Abu-Assi is associate editor of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed.
Supplementary data associated with this article can be found in the online version, at available https://doi.org/10.1016/j.rec.2021.03.006