To the Editor:
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare entity, first described in 1995 by Leenhardt et al,1 which is characterized by repeated syncope and sudden death due to polymorphic ventricular arrhythmia in childhood, coinciding with physical or emotional exertion.
The symptoms start after 4 years of age, with diagnosis usually occurring around age 10-15; it is rarely diagnosed after age 30. Because of the self-limiting nature of most arrhythmia episodes, many of these patients are diagnosed as having seizures. This self-limiting characteristic should be taken into consideration when a defibrillator is implanted, with proper programming to prevent unnecessary shocks that favor arrhythmia, as occurred in the case described.
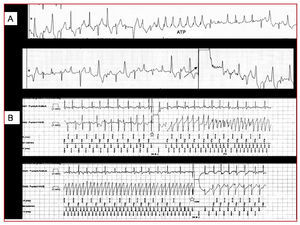
We describe a 38-year-old woman, with mild to moderate mental disability and living at a nursing care residence, who had been diagnosed with epilepsy at age 4 and was on phenytoin therapy. In the past 2 years she had presented atrial fibrillation (AF) crises, which were controlled with 200 mg/day of oral amiodarone. On the day of admission, she presented palpitations due to atrial fibrillation, which were treated with intravenous amiodarone; at discharge she presented syncope, with torsade de pointes that reverted with shock (Figure 1A), restarted, and self-limited on several occasions. After spontaneous stabilization, she was admitted to the intensive care unit (ICU).
Figure 1. A: torsade de pointes treated by shock, which reinitiates spontaneously. B: start (star) of isoproterenol infusion, with onset of considerable ectopy, ventricular bigeminy, and runs of polymorphic ventricular tachycardia (arrow). The prohibition symbol marks the end of the infusion, with rhythm normalization.
The phenytoin concentration was within the therapeutic range, and no ion alterations were observed. The QT interval was normal at all times. Coronary angiography and cardiac MRI were normal. During ICU monitoring, a tendency toward exaggerated sinus bradycardia was observed, even though all medication had been withdrawn 1 week earlier; frequent ventricular extrasystole was also observed in relation to emotional stress.
The isoproterenol test showed the reproducible appearance of ventricular ectopy and ventricular bigeminy, with polymorphic tachycardia developing when the infusion was continued. The arrhythmias disappeared when perfusion ceased (Figure 1B), thus confirming the CPVT pattern described by Leenhardt et al.1
The patient was treated with high doses of beta-blockers and an automatic implantable defibrillator was implanted, based on the evidence of ventricular fibrillation (VF) and to prevent bradycardia induced by beta-blocker therapy. In particular, a long atrioventricular interval was programmed to prevent ventricular pacing.
At 15 days, she returned to the hospital because of multiple discharges. Antitachycardia therapies (ATT) over bidirectional tachycardia (Figure 2A) were observed during monitoring, but seen to have no effect whatsoever. Device testing revealed multiple ATTs and shocks over a possibly bidirectional ventricular tachycardia, given the changing morphology of the electrograms. On several occasions, an episode of VF occurred after the shock and was treated with another shock (Figure 2B). Finally, after reprogramming the defibrillator to leave only one VF window as of 220 bpm and increasing the number of R-R intervals that should meet the frequency criteria, the patient remained shock-free.
Figure 2. A: bidirectional tachycardia that is not suppressed by antitachycardia therapy or shocks (arrow). B: electrocardiograms stored by the automatic implantable defibrillator reveal polymorphic tachycardia in the ventricular channel and the triggering of a shock (star) that causes ventricular fibrillation, suppressed by a second shock in the lower tracing.
The case has two features of interest: age of presentation and importance of properly programming the automatic implantable defibrillator. The condition is usually diagnosed around 15 years of age; in this case the diagnostic delay may be explained by the presence of 2 factors that protected the patient from further episodes, namely, phenytoin therapy and the sedentary lifestyle of her place of residence, which reduced adrenergic tone.
In terms of programming, ATTs are not useful and use up the battery unnecessarily, since the mechanism of ectopy and bidirectional tachycardia would apparently be related to activity triggered by late postdepolarizations.2,3 Lastly, most episodes of polymorphic tachycardia are self-limiting and asymptomatic. Therefore, programming short detection intervals for a VF window may produce unnecessary shocks that increase the adrenergic situation and lead to further proarrhythmia, possibly exhausting all therapeutic options and jeopardizing the life of the patient. The defibrillator should be programmed using a single-area FV approach with long detection times, such that the shock is only released when the polymorphic tachycardia is true FV.4