Diagnosis of arrhythmogenic right ventricular cardiomyopathy (ARVC) can be challenging. Recent evidence indicates that the natural history of this disease includes a first concealed phase, characterized by acute exacerbations of myocardial inflammation and life-threatening ventricular arrhythmias, occurring prior to the onset of classical characteristics and contributing to its pathogenesis and progression.1 This has been demonstrated by reports of ARVC presenting as recurrent myocarditis-like episodes in young patients with evidence of myocardial inflammation on cardiac magnetic resonance.2 Instead of the classical replacement in this disease of myocytes by fibrous or fibroadipose tissue in the right ventricular (RV) myocardium,3 inflammatory infiltrates can often be seen in affected areas.4 This article intends to illustrate this association, making a compelling argument for a thorough investigation of the RV in young patients presenting with ventricular arrhythmias and signs of active or past myocarditis.
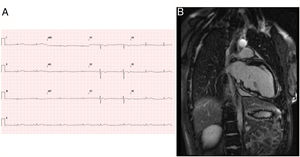
Patient 1, a previously healthy girl, presented at the age of 15 years with aborted sudden cardiac death during competitive sports. Rhythm was pulseless ventricular tachycardia. The baseline electrocardiogram (ECG) showed low voltage and T wave inversion in the right leads (figure 1), thought to be normal for her age. Her father had the same T wave pattern, but family history was otherwise not relevant. One week before the current event, she was diagnosed with tracheobronchitis, with 1 day of fever. During the current admission, she progressed well. Twenty-four hour Holter monitoring showed isolated, polymorphic ventricular ectopic beats (28 beats/h). Cardiac magnetic resonance revealed indirect signs of active inflammation with enhanced spontaneous left ventricular (LV) myocardial SSFP signal, and multiple locations of subepicardial late gadolinium enhancement (LGE), which were more evident at the inferior LV wall (figure 1). LV ejection fraction (EF) was 58%, and end-diastolic volume was normal (85mL/m2). RV ejection fraction was 48%, and end-diastolic volume was at the upper limit of normal (108mL/m2). Mild dyskinesia of the right ventricular outflow tract (RVOT) was noted (), but was not consensual. At the time, a presumptive diagnosis of acute myocarditis was made, despite negative etiologic investigation. An implantable cardioverter-defibrillator (ICD) was implanted. Genetic screening by a sequencing method revealed a previously unknown variant in heterozygosis: c.1840delC in the PKP2 gene, causing protein truncation, diagnostic for ARVC. The father is a gene carrier, exhibiting only the ECG abnormalities. His extended family was not studied. The patient has been followed-up and has been well for 2 years.
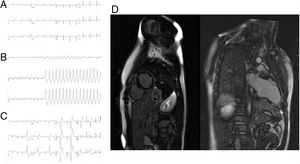
Patient 2, a 13-year-old boy, was referred due to ventricular ectopia of left bundle branch block morphology in the last 2-yearly ECGs performed for competitive sports practice. ECGs also showed T wave inversion in the right precordial leads (figure 2), considered normal for his age. The family history was negative and the patient was previously healthy except for an episode of oppressive chest pain 2 years earlier lasting a few days and preceding the appearance of ectopia. Upon current evaluation, he mentioned light-headedness and palpitations during sports. The echocardiogram revealed mild RV dilation. Exercise testing elicited nonsustained RVOT ventricular tachycardia (figure 2) and multiple isolated RVOT ventricular ectopic beats (figure 2). Cardiac magnetic resonance showed normal LV dimension and function, but extensive subepicardial LGE (figure 2). The RV was mildly dilated (115mL/m2), and EF was 39%, with mild apex and RVOT dyskinesia (). Due to the patient's previous history of chest pain, LGE pattern and RV dyskinesia, doubts were elicited about the correct diagnosis: scar tissue from a previous episode of myocarditis and/or ARVC manifested by a previous inflammatory exacerbation. Beta-blocker therapy was instituted. While awaiting implantable cardioverter-defibrillator implantation, the patient experienced an episode of sustained RVOT tachycardia. Follow-up electrophysiologic study induced and successfully ablated this tachycardia. Another form of faster ventricular tachycardia originating from the apex was induced but not sustained sufficiently to allow mapping. A subcutaneous implantable cardioverter-defibrillator was implanted. High resolution signal-averaged ECG showed late potentials. Genetic testing by a sequencing method revealed a previously undescribed variant in heterozygosis: c.224-2T>C in the PKP2 gene, leading to protein truncation and diagnosis of ARVC. First-degree relatives declined study. The patient has been medicated with sotalol, followed-up, and has been asymptomatic for 1 year.
Patient 2. A: baseline ECG with T wave inversion at the right precordial leads. B: ventricular tachycardia during exercise testing with inferior axis. C: ventricular ectopic beats with RVOT origin. D: cardiac magnetic resonance showing extensive subepicardial late gadolinium enhancement, especially at the inferolateral left ventricular wall.
These cases illustrate how ARVC can present early in life with life-threatening ventricular arrhythmias and inferolateral subepicardial LGE. This is a common LGE pattern both in myocarditis5 and ARVC with LV involvement.1 Therefore, myocarditis, which is more prevalent at such a young age, may be precipitously diagnosed. Careful consideration of other diagnostic cues, such as RV size and regional contractility in our patients, may lead to comprehensive genetic testing6 and uncover the underlying disease. In fact, both patients met task force criteria3 (3 major+1 minor and 2 major+2 minor criteria, respectively). Achieving a correct diagnosis of ARVC has major implications for future management and familial risk stratification.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2019.10.007