An 86-year-old man, with previous coronary artery disease (recanalization of chronic total occlusion of the right coronary artery) and left ventricular ejection fraction of 45%, presented with symptomatic severe aortic stenosis. Surgical risk was considered high (Society of Thoracic Surgery score 8.1%) and a decision was made to perform transcatheter aortic valve replacement (TAVI).
Preprocedural multislice computed tomography (MSCT) showed diffuse and severe calcified peripheral artery disease. The distal abdominal aorta showed mural thrombosis and calcification, with an effective diameter of 8.0mm. Transfemoral access was prevented by diffuse heavily calcified bilateral iliac and femoral artery stenosis, with minimum lumen diameter of 4.5mm on the right and 4.3mm on the left. Axillary artery access was unsuitable due to significant peripheral artery disease. Due to previous thoracic radiation and chronic obstructive pulmonary disease, the transapical and direct aortic approaches were not considered as optimal alternatives to the femoral route for this patient and so the possibility of transcaval approach was evaluated. Preprocedural MSCT confirmed eligibility for transcaval TAVI, ruling out anatomical constraints and limiting conditions.1
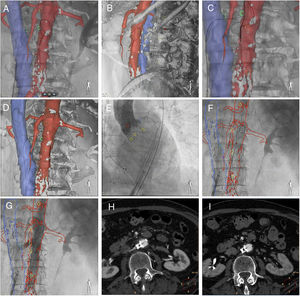
The technique of transcaval access and closure, which has been described previously,2–4 and retrograde transcatheter aortic valve replacement using a standard transfemoral technique and MSCT-fluoroscopy fusion imaging guidance are shown in figure 1. After simultaneous caval and aortic angiograms (), a 3D segmented model with associated landmarks (green circles marked the planned transverse puncture site, yellow circles marked vertebrae) was registered on fluoroscopy: anteroposterior (figure 1A) and lateral (figure 1B) fluoroscopic views confirming correct position of the catheter in the vena cava and snare in the aorta. An electrified guidewire crossed the aorta wall at the planned level (green circles, figure 1C), and was trapped in the aortic lumen by the snare (figure 1D, ). CoreValve Evolut PRO 29mm was implanted (yellow circles marked the native aortic annulus plane, figure 1E). To close the transcaval access, an Amplatzer duct occluder device was deployed using a deflectable catheter to achieve perpendicular deployment to the aorta during pullback (figure 1F, ). The immediate aortic angiogram after closure showed minimal aortocaval flow (figure 1G). The final aortogram showed no residual aortocaval flow or contrast extravasation (). Postprocedural MSCT showed an aortocaval fistula and ruled out other vascular complications of transcaval access (figure 1H). Follow-up MSCT after 30 days demonstrated complete closure of the fistula (figure 1I).
To the best of our knowledge, the use of MSCT-fluoroscopy fusion imaging (HeartNavigator system; Philips Healthcare, The Netherlands) to facilitate transcaval TAVI has not been previously reported. MSCT is crucial for assessment of eligibility for transcaval TAVI and to determine the optimal caval and aortic puncture site. During transcaval TAVI, MSCT-fluoroscopy fusion imaging determines the optimal fluoroscopy angulations, leads to a reduction in fluoroscopy time and volume contrast and improves procedure safety, since it provides a 3D “road map” for the intervention.
At the present time, MSCT is the gold standard imaging technique for TAVI procedural planning. MSCT-fluoroscopy fusion imaging provides an alternative tool for procedural planning and guidance.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2019.10.009