Arterial stiffness is an important manifestation of subclinical organ damage linked to aging and provides an established marker of cardiovascular disease.1 Carotid-femoral pulse wave velocity (cfPWV), measured invasively or noninvasively, is the gold standard indicator of arterial stiffness.
Some studies have shown that aortic pulse wave velocity (PWV) has an additive value in predicting cardiovascular events above and beyond blood pressure and other traditional cardiovascular risk factors, including those combined into the European Systematic COronary Risk Evaluation (SCORE) and the Framingham risk score.2,3 Furthermore, PWV is increasingly being considered in hypertension clinical guidelines. For example, the 2018 European Society of Cardiology (ESC)/European Society of Hypertension (ESH) guidelines for the management of arterial hypertension included PWV as an index of aortic stiffness and underlying arteriosclerosis as part of the assessment of hypertension-mediated organ damage.3 Further, a scientific statement from the American Heart Association has developed recommendations for improving and standardizing vascular research on arterial stiffness.4
In addition, the ESC Working Group on peripheral circulation (endorsed by the Association for Research into Arterial Structure and Physiology [ARTERY] Society) stated that cfPWV meets most, but not all, of the required criteria to be considered a clinical surrogate endpoint for cardiovascular disease.5 Nevertheless, its validation as such is already well on its way. One of these criteria, according to the ESC and ARTERY Society,5 is the availability of reference values or, at least, cutoff values, for the assessment of this parameter as a vascular biomarker. Currently, the clinical utility of PWV (and even more of other arterial stiffness parameters) is still limited due to the relative scarcity of normative data (from normotensive individuals without any major cardiovascular risk factors), reference data (from participants with cardiovascular risk factors) and data for each particular measuring device.
One major study reported normal and reference values for cfPWV from 13 centers across 8 European countries.6 Because there are substantial differences in reference values among methodologies and populations, this pooling study standardized methodology and used data from a wide population. Standardization, however, did not completely remove differences in techniques.6 For this and other reasons, these normative and reference values are applicable mainly to settings where measurements are performed with the same methodologies as those used in the pooling. Thus, efforts to establish “local” PWV reference values are worth undertaking even if the results are only valid for a specific population and 1 PWV methodology.
Other studies have reported norms or reference values for arterial stiffness parameters, mainly in countries, settings, and ethnicities not included in the aforementioned pooling study. They provide local reference values for one or more PWV parameters in adults, generally confirming the strong impact of age and blood pressure on arterial stiffness.7,8
Regarding Spain, a handful of previous studies have presented normative or reference values for arterial stiffness parameters for diverse populations.9,10 Elosua-Bayés et al.9 reported normative values of the cardio-ankle vascular index (CAVI) based on a general population of Girona, a Mediterranean province in Spain. CAVI is an arterial stiffness parameter that, unlike cfPWV, is independent of blood pressure at the time of measurement. About 40% of the 2613 adults examined had a CAVI> 9, which was positively associated with age, most other common cardiovascular risk factors, and coronary risk. Worth mentioning is that this score was reached by 60% of certain subgroups not considered to be at high risk. This suggests that this index could be useful in improving risk stratification. Sánchez-Martínez et al.,10 reported normal and reference values for PWV specific to community-dwelling older Spaniards (n=1824), a population for which this parameter is of particular importance. That study used a simple-to-use brachial cuff-based oscillometric device (Mobil-O-Graph), whose aortic PWV values (estimating cfPWV) have been validated and have shown good reproducibility, but more evidence is still needed.4
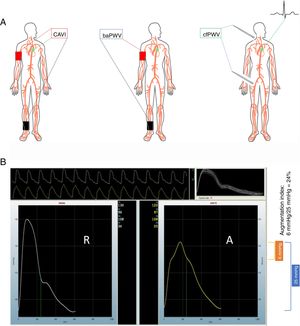
In a recent article published in Revista Española de Cardiología, Gómez-Sánchez et al., 11 present a timely, comprehensive analysis of the distribution and correlates of as many as 4 parameters of arterial stiffness. These 4 measures, reflecting different vascular beds, were obtained from a random sample recruited in 5 primary care health centers and composed of 501 adults aged 35 to 74 years and apparently cardiovascular disease-free. These arterial stiffness parameters were measured using validated devices and included CAVI, brachial-ankle PWV (baPWV) (both measured by the VaSera device), cfPWV and the central augmentation index (both measured with the SphygmoCor device). The figure 1 shows graphically how these values are obtained. CAVI links heart sounds with pressure input from brachial and ankle cuffs to develop an index of vascular stiffness that reflects the stiffness in the aorta, femoral and tibial arteries expressed, as a unitless value called the “β”.12 baPWV detects the time elapsed between pulsation in the brachial cuff and pulsation in the ankle cuff. The pathway of pulse travel is derived from the distance between the sternal notch and the ankle cuff minus the distance from the sternal notch to the brachial cuff. This brachial cuff pulsation is used as a surrogate for the aortic pulse. The difference in timing of pulse travel as detected in the brachial cuff vs the ankle cuff provides the time estimate. The distance divided by the time of each pulse wave provides a measure of PWV, which reflects stiffness by combining both the aortic (central) and femoral/tibial (peripheral) circulations.13 cfPWV is obtained by measuring pulse arrival at the carotid artery and the femoral artery. When done in sequence, an electrocardiogram is used to “gate” the time elapsed from the peak of the R-wave to the arrival of the pulse wave at the carotid, or the femoral site. When the carotid and femoral sites are recorded simultaneously, an electrocardiogram is not necessary, and the PWV is determined by dividing the distance the pulse travels by the time elapsed between the onset of each carotid pulse waveform and each femoral waveform.4
A: common methods to measure arterial stiffness. Left: carotid-ankle vascular index (CAVI). Center: brachial-ankle pulse wave velocity (baPWV). Right: carotid-femoral pulse wave velocity (cfPWV). Pictured on the right is the use of a tonometer to obtain the vascular waveform; however, a cuff can also used in both the carotid and the femoral locations. B: derivation of central augmentation index. The panel has 2 assembled waveforms labeled radial (“R”) and aortic (“A”). The operator records 10seconds of radial waveform shown in the upper portion of panel B. The waveform is calibrated by entering the brachial blood pressure at the time of measurement. The software uses an algorithm to estimate the central pressure waveform using the radial waveform data and displays the central aortic pressure waveform on the right. In this example, the brachial blood pressure was 130/96mmHg. The central aortic pressure is 122/97mmHg. The pulse pressure in the central aortic waveform, shown in the blue bracket, is 25mmHg (calculated as the systolic minus the diastolic value). The central aortic waveform upstroke clearly changes slope at 116mmHg, marked by a green dot on the right border of the aortic waveform. The systolic value (122mmHg) minus the value at the deflection point (116mmHg) yields a difference of 6mmHg shown in the orange bracket, representing the augmentation pressure experienced by the left ventricle when completing systole. This augmentation pressure results from the backward-traveling pressure wave, which arrives at the left ventricle in late systole and contributes to the final central pressure waveform. The central augmentation index is the ratio of this augmented pressure divided by the aortic pulse pressure.
An added value of the study11 is that the authors examined the relationship between these 4 parameters and major cardiovascular risk factors.
Whereas several indices indeed reflect arterial stiffness to a degree, the best measure is PWV, whether by invasive or noninvasive means, as recommended in an American Heart Association (AHA) scientific statement and European expert consensus documents.1,4 Further, both the AHA document,4 and the European position paper of the ESC and the ARTERY Society5 recommended the assessment of cfPWV given the predominance of prospective evidence supporting the superiority of this vascular biomarker for predicting outcomes. However, both risk refinement, ie, reclassification in a higher/lower risk stratum, and relevant therapeutic decisions can be made based on 1 or more biomarkers. Assessment of different parameters has 2 advantages: first, they may offer valuable complementary information and, second, the weight placed on one parameter over another may be dictated by the clinical setting and comorbidities.
The above-mentioned study 11 reported lower cfPWV values compared with those from the European pooling6 and the Spanish study (comparing specifically older people).10 These discrepancies may reflect methodological differences across studies (eg, the use of different devices) but also disparities in the prevalence of cardiovascular risk factors or other determinants of arterial stiffness in the populations examined. For example, the authors used applanation tonometry to measure cfPWV,11 whereas the Spanish study10 used an oscillometry-based device that provides only an estimate of cfPWV. Nevertheless, reference values for the tonometry-based device, the gold standard, are bound to be especially useful for specialists with access to the more sophisticated technique, whereas oscillometry-based reference values are more likely to be used by primary care physicians as they incorporate the simpler, albeit validated, oscillometric technique.
It is worth mentioning that, despite the use of the same measurement device, the CAVI mean values reported by the authors11 were lower than those observed in a population from a Spanish Mediterranean region.9 Thus, factors other than methodology are probably contributing to these variations. Regarding sex differences, the authors argue that the higher CAVI and cfPWV values found in men11 could be attributable to the protective effect of estrogens enjoyed by premenopausal women despite their having higher degree of arterial stiffness intrinsically. A major strength of this study11 is that it provides one of the scarce sets of baPWV population data based on Caucasian individuals for comparison. Lastly, in this study,11 hypertension was the only cardiovascular risk factor independently associated with all 4 arterial stiffness parameters.
Beyond average reference values, a cfPWV> 10 m/s is considered a conservative estimate of significant alterations of aortic function in middle-aged hypertensive patients.3 The study in question11 did not calculate the proportion of adults at higher risk according to any parameter threshold. Some authors have calculated early vascular aging (EVA), defined as PWV ≥ 97.5th percentile of z-score for mean PWV values adjusted for age (using normative European reference values as comparators).8 Although an attractive approach due to its simplicity, risk estimation based on fixed thresholds has several limitations. For instance, the existence of a relatively continuous relationship between risk and cfPWV, and the failure to consider factors such as the transient elevation of mean arterial pressure, may confound cfPWV values because of the nonlinear stiffness of the aortic wall.4
By and large, the availability of normative and reference arterial stiffness values derived from populations in a particular region with a particular measuring device seems desirable. However, as experience taught us with similar issues such as cardiovascular risk charts, this degree of specificity should be curbed and the inflation of reference values should be avoided at the risk of confusing physicians, and other potential users, and reducing its use. Having said that, every new single or combined vascular biomarker with its corresponding reference values should be welcome from a research point of view and, consequently, the validation of corresponding parameters is a desirable sound practice. At the same time, speaking now from the clinical perspective, guidelines should be as reliable and simple as possible if the aim is to incorporate, use, and interpret any of these biomarkers in routine practice.
Some perspectives deserve further comment. Today, due to our ability to measure 24-hour average PWV, we know it follows a typical circadian rhythm. Thus 24-hour ambulatory pulse wave analysis shows promise as a tool for the evaluation of vascular biomarkers in daily life conditions.14 Moreover, ambulatory PWV has shown associations with cardiovascular events and all-cause mortality in some settings; however, we need long-term outcome studies to test the predictive value of this parameter over and beyond conventional blood pressure.14 Some researchers have incorporated community pharmacies to measure 24-hour ambulatory blood pressure and even arterial stiffness, which may facilitate more accurate stratification of patients’ cardiovascular risk, thus allowing for a greater personalization of the physician's intervention.14,15 Unfortunately, the term “arterial stiffness” is practically nonexistent in Spaińs public health administrations programs.15 We hope this commentary will be a step forward toward improving this situation.
Two final thoughts: first, we should consider the possibility that the ideal arterial stiffness parameter may differ for each patient, a concept that should be explored further. Second, the promise of therapeutic decisions driven by vascular biomarkers should be realized and validated through randomized clinical trials.5
FUNDINGJ.R. Banegas has received support from Fondo de Investigación Sanitaria (FIS) grants no. PI13/02321 and 16/01460 (Instituto de Salud Carlos III and FEDER/FSE) and CIBERESP, and Cátedra UAM de Epidemiología y Control del Riesgo Cardiovascular (#820024). R.R. Townsend has nothing to declare. The funding agencies had no role in study design, data analysis, interpretation of results, manuscript preparation or in the decision to submit this manuscript for publication.
CONFLICTS OF INTERESTNone declared.