In recent years, increasing evidence has emerged to support the distinct biological behavior of patients with heart failure (HF) in the upper ranges of left ventricular ejection fraction (LVEF). Indeed, among patients with HF and preserved ejection fraction (HFpEF), those with higher LVEF have been termed as having “supranormal” ejection fraction (HFsnEF).1–3
There are well-known differences among patients with reduced, mid-range, and preserved ejection fraction.1,3 However, the factors associated with those patients with higher systolic function remain poorly understood. Along this line, heightened inflammatory activity has emerged as a crucial pathophysiological mechanism and potential therapeutic target in HFpEF.4 For instance, an ongoing trial is evaluating the efficacy of ziltivekimab vs placebo in patients with ambulatory HF, LVEF >40%, and high-sensitivity C-reactive protein (hs-CRP) >2mg/L (NCT05636176). No prior studies have evaluated the inflammatory status profile along the continuum of LVEF, especially when LVEF ≥50%. In this study, we aimed to examine whether circulating hs-CRP at presentation differs along the continuum of LVEF in patients with acute HF (AHF) and LVEF ≥ 50%.
We conducted a retrospective study of an ongoing multicenter registry of patients admitted with AHF from January 2010 to January 2021 that enrolled 5246 patients. Patients with evidence of LVEF <50% during hospitalization (n=2433), evidence of infection at admission (n=113), missing values of hs-CRP (n=312), early deaths without assessment of LVEF (n=38) were excluded from this analysis. No patients received inotropes at presentation. The final study sample included 2350 patients. Clinical and biochemical characteristics, including hs-CRP, were assessed at presentation. Echocardiographic assessments, including LVEF, were performed during hospitalization (72±24hours after admission). LVEF was assessed by 2-dimensional echocardiography using the Simpson method. The association between hs-CRP and LVEF was evaluated along the continuum of LVEF or dichotomized (< 65% vs ≥65%). Continuous variables are presented as mean±standard deviation or median (percentile 25% to percentile 75%), and their differences across LVEF quartiles were tested using ANOVA or Kruskal-Wallis tests. Discrete variables were presented as numbers (percentages), and differences were examined using the chi-square test. The multivariable relationship between hs-CRP along the continuum of LVEF and <65% vs ≥ 65% was examined through multivariate linear regression analysis and logistic regression, respectively. Candidate covariates included in the multivariate models were based on biological plausibility. The linearity assumption for all continuous variables was simultaneously tested, and the variable transformed, if appropriate, with fractional polynomials. The contribution of the covariates to the variability of the linear regression model was evaluated by R-square, and the discriminative ability of the multivariate model was assessed by the area under the receiver operating characteristic curve. The final models included the following covariates: age, sex, first admission, prior stable New York Heart Association (NYHA) class, ischemic heart disease, Charlson comorbidity index, systolic and diastolic blood pressure, heart rate, atrial fibrillation, creatinine, N-terminal pro-B-type natriuretic peptide (NT-proBNP), left atrial diameter, left ventricular end-diastolic diameter, and tricuspid annular plane systolic excursion (TAPSE).
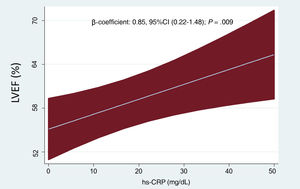
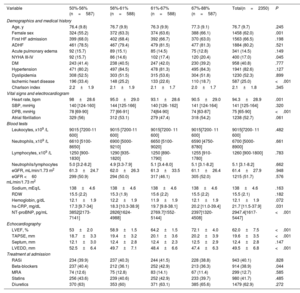
The mean age was 76.7±9.7 years, and 1458 patients (62%) were women. The proportion of patients with ischemic heart disease was 25%. The mean LVEF was 62±7%, and 876 (37.3%) showed LVEF ≥ 65%. The median of hs-CRP on admission was 19.7mg/dL (10.5-37.9). Baseline characteristics across LVEF quartiles are presented in table 1. Patients in the upper quartiles of LVEF were more frequently women and were less likely to have a history of ischemic heart disease. Likewise, they showed lower heart rate, diastolic blood pressure, NT-proBNP, and left ventricular end-diastolic diameters. Conversely, these patients showed a higher proportion of NYHA class III/IV before admission, higher TAPSE, and higher hs-CRP values. Inferential multivariate linear regression analysis confirmed the significant and positive association between higher hs-CRP and LVEF. This relationship was linear (figure 1). The logistic multivariate regression analysis also confirmed higher hs-CRP as a predictor of LVEF ≥ 65%. Indeed, per each 1mg/dL increase of hs-CRP, the odds increased by 22% (odds ratio [OR], 1.22, 95% confidence interval [95%CI], 1.01-1.48; P=.046). The R-square (linear regression) and the area under the receiving operating curve (logistic regression) were 0.33 and 0.706, respectively.
Baseline characteristics across LVEF quartiles
| Variable | 50%-56%(n=587) | 56%-61%(n=588) | 61%-67%(n=588) | 67%-88%(n=587) | Total(n=2350) | P |
|---|---|---|---|---|---|---|
| Demographics and medical history | ||||||
| Age, y | 76.4 (9.8) | 76.7 (9.9) | 76.3 (9.9) | 77.3 (9.1) | 76.7 (9.7) | .245 |
| Female sex | 324 (55.2) | 372 (63.3) | 374 (63.6) | 388 (66.1) | 1458 (62.0) | .001 |
| First HF admission | 399 (68.0) | 402 (68.4) | 392 (66.7) | 370 (63.0) | 1563 (66.5) | .198 |
| ADHF | 461 (78.5) | 467 (79.4) | 479 (81.5) | 477 (81.3) | 1884 (80.2) | .521 |
| Acute pulmonary edema | 92 (15.7) | 89 (15.1) | 85 (14.5) | 75 (12.8) | 341 (14.5) | .149 |
| NYHA III-IV | 92 (15.7) | 86 (14.6) | 102 (17.4) | 120 (20.4) | 400 (17.0) | .045 |
| DM | 243 (41.4) | 238 (40.5) | 247 (42.0) | 230 (39.2) | 958 (40.8) | .777 |
| Hypertension | 471 (80.2) | 497 (84.5) | 478 (81.3) | 495 (84.3) | 1941 (82.6) | .127 |
| Dyslipidemia | 308 (52.5) | 303 (51.5) | 315 (53.6) | 304 (51.8) | 1230 (52.3) | .899 |
| Ischemic heart disease | 196 (33.4) | 148 (25.2) | 133 (22.6) | 110 (18.7) | 587 (25.0) | <.001 |
| Charlson index | 2.2±1.9 | 2.1±1.9 | 2.1±1.7 | 2.0±1.7 | 2.1±1.8 | .345 |
| Vital signs and electrocardiogram | ||||||
| Heart rate, bpm | 98±28.6 | 95.0±29.0 | 93.1±28.6 | 90.5±29.0 | 94.3±28.9 | .001 |
| SBP, mmHg | 140 [124-160] | 144 [125-166] | 140 [126- 162] | 141 [124-164] | 141 [125-164] | .320 |
| DPB, mmHg | 78 [69-90] | 77 [66-91] | 74[64-90] | 74 [63-87] | 75 [65-90] | <.001 |
| Atrial fibrillation | 329 (56) | 312 (53.1) | 279 (47.4) | 318 (54.2) | 1238 (52.7) | .061 |
| Blood tests | ||||||
| Leukocytes, x109 /L | 9015 [7200-11 600] | 9015 [7200-11 600] | 9015[7200- 11 600] | 9015[7200- 11 600] | 9015[7200- 11 600] | .482 |
| Neutrophils, x109 /L | 6610 [5100-8650] | 6900 [5000-9210] | 6650 [5100-9020] | 6590 [4750-8780] | 6700 [5000-8900] | .661 |
| Lymphocytes, x109 /L | 1250 [900-1830] | 1290 [935-1820] | 1250 [890-1790] | 1255 [910-1780] | 1260 [900-1800] | .783 |
| Neutrophils/lymphocytes | 5.0 [3.2-8.2] | 4.9 [3.3-7.9] | 5.1 [3.4-8.0] | 5.1 [3.1-8.2] | 5.1 [3.1-8.2] | .662 |
| eGFR, mL/min/1.73 m2 | 61.3±24.7 | 62.0±26.3 | 61.3±33.5 | 61.1±26.4 | 61.4±27.9 | .948 |
| eGFR <60 mL/min/1.73 m2 | 299 (50.9) | 294 (50.0) | 317 (46.1) | 305 (52.0) | 1215 (51.7) | .576 |
| Sodium, mEq/L | 138±4.6 | 138±4.6 | 138±4.6 | 138±4.6 | 138±4.6 | .163 |
| RDW | 15.5 (2.2) | 15.3 (1.9) | 15.6 (2.2) | 15.5 (2.2) | 15.5 (2.1) | .182 |
| Hemoglobin, g/dL | 12.1±1.9 | 12.2±1.9 | 11.9±1.9 | 12.1±1.9 | 12.1±1.9 | .072 |
| hs-CRP, mg/dL | 17.3 [9.7-34] | 18.3 [10.3-38.9] | 19.7 [9.8-38.1] | 20.2 [11.0-39.4] | 21.7 [11.5-37.9] | .031 |
| NT-proBNP, pg/mL | 3852[2173-7141] | 2826[1624-4988] | 2769.7[1552- 5144] | 2397[1320- 4508] | 2947.4[1617-5447] | <.001 |
| Echocardiography | ||||||
| LVEF, % | 53±2.0 | 58.9±1.5 | 64.2±1.5 | 72.1±4.0 | 62.0±7.5 | <.001 |
| TAPSE, mm | 18.7±3.3 | 19.4±3.2 | 20.1±3.6 | 20.2±3.9 | 19.6±3.5 | <.001 |
| Septum, mm | 12.1±3.0 | 12.4±2.8 | 12.4±2.3 | 12.5±2.9 | 12.4±2.8 | .147 |
| LVEDD, mm | 52.5±6.4 | 49.7±7.1 | 48.4±6.6 | 47.4±6.3 | 49.5±6.8 | <.001 |
| Treatment at admission | ||||||
| RASi | 234 (39.9) | 237 (40.3) | 244 (41.5) | 228 (38.8) | 943 (40.1) | .828 |
| Beta-blockers | 237 (40.4) | 212 (36.1) | 252 (42.9) | 213 (36.3) | 914 (38.9) | .044 |
| MRA | 74 (12.6) | 75 (12.8) | 83 (14.1) | 67 (11.4) | 299 (12.7) | .585 |
| Statins | 256 (43.6) | 239 (40.6) | 252 (42.9) | 233 (39.7) | 980 (41.7) | .485 |
| Diuretics | 370 (63) | 353 (60) | 371 (63.1) | 385 (65.6) | 1479 (62.9) | .272 |
ADHF, acute decompensated heart failure; DM, diabetes mellitus; DPB, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HF, heart failure; hs-CRP, high-sensitivity C-reactive protein; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; RASi, renin-angiotensin-system inhibitors; RDW, red cell distribution width; SBP, systolic blood pressure; TAPSE, tricuspid annular plane systolic excursion.
Data presented as No. (%), mean±standard deviation, or median [Q1-Q3].
Following the comorbidity-inflammation paradigm in HFpEF, the current work shows a significant association between higher LVEF levels and higher hs-CRP values in AHF. This paradigm postulates that a greater comorbidity burden will induce systemic vascular inflammation, leading to endothelial dysfunction, myocardial fibrosis, high diastolic stiffness, and clinical HF.4 We postulate that the greater comorbidity burden and immunoinflammatory activation increases oxygen demand and that the heart will initially compensate by increasing systolic function. As the situation advances, encompassing increased myocardial fibrosis, this compensatory mechanism will prove insufficient, leading to progression of HF.
Our study has several limitations. First, this is a retrospective single-center study and extrapolation of the current findings to other scenarios requires confirmation. Second, we did not explore the association between higher HFsnEF and hs-CRP values and adverse clinical outcomes. Third, we excluded patients with infections on admission; however, we cannot exclude subclinical infection or other proinflammatory confounders. Fourth, we had no data on weight, height, cytokines, other acute-phase reactants, liver function, coagulation, or troponins in any of the patients. Finally, with the current data, we cannot infer causality or unravel the biological mechanisms behind it.
FUNDINGThis work was supported in part by grants from Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV) (grant numbers 16/11/00420 and 16/11/00403).
ETHICAL CONSIDERATIONSThe study conformed to the principles outlined in the Declaration of Helsinki and was approved by the local ethics committee (Hospital Clínico Universitario de Valencia). All patients previously gave informed consent. SAGER guidelines have been considered.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence tool was used in the preparation of this work.
AUTHORS’ CONTRIBUTIONSThe authors have no other funding, financial relationships, or conflicts of interest to disclose relative to this work.
CONFLICTS OF INTERESTNone.